Rotavirus outbreak linked to poor hygiene practices at a babies’ home in Mpigi District, Uganda, July─August 2023
Authors: Dorothy Aanyu1*, Priscilla Atim1, Brian Kibwika1, Benigna Namara1, Samuel Gidudu1, Benon Kwesigwa1, Doreen N. Gonahasa1, Alex Riolexus Ario1; Institution affiliations, 1Uganda Public Health Fellowship Program, Uganda National Institute of Public Health, Kampala, Uganda; Correspondence*: Tel: +256774009185, Email: daanyu@uniph.go.ug
Summary
Background: In Uganda, the rotavirus vaccine for children <5 years was introduced in 2018. By 2022, rotavirus as a cause of acute diarrhea among children had decreased by 40%. On August 10, 2023, the Uganda Ministry of Health was notified of a suspected rotavirus disease outbreak at a babies’ home in Mpigi District. We investigated to determine the magnitude and identify possible exposures associated with the outbreak.
Methods: We described a suspected case as acute onset of diarrhea plus ≥1 of the following: vomiting, fever, lethargy or loss of appetite in a child or staff member at the babies’ home from July 27─ Aug 20, 2023 OR A positive rotavirus RDT result in an asymptomatic child or staff member. A confirmed case was a suspected case with a positive ELISA test for rotavirus A. We identified cases by reviewing health records and conducting mass RDT testing. We conducted descriptive epidemiology, staff interviews, and an environmental assessment to determine possible sources of exposure.
Results: Among the 44 children and 59 adults at the home, we line-listed 23 case-patients (21 children and 2 adults). Of these, 6 were confirmed and 13 were suspected (1 death). Symptom onsets ranged from August 3─17, 2023. Twenty (87%) case-patients were fully vaccinated against rotavirus. Attack rates were highest in children aged 13-18 months (86%) followed by the 7-12 months ages group (83%). No cases were reported in the children >2 years. The environmental assessment indicated poor hand hygiene practices among the caregivers. Of the caregivers interviewed, 9/23 used disposable gloves, 4/23 washed their hands after cleaning vomitus and fecal spills and 9/23 washed their hands after washing the childrens’ potties.
Conclusion: The outbreak was caused by rotavirus, with spread likely facilitated by poor hygiene practices. We instituted infection prevention and control measures and decontaminated the babies’ home.
Background
Rotaviruses are the leading cause of severe, diarrhea in children under the age of five years globally. They are spread through the fecal-oral route and during active infection high concentrations are shed in the stool and vomitus of infected individuals. Transmission may also occur through contaminated food, water, hands, and surfaces. Upon entry into a human body, rotavirus disrupts the function of the small intestinal epithelium, causing malabsorption of water and resulting in watery diarrhea.
Once infected, it takes two to three days for a child to develop signs and symptoms. The World Health Organization (WHO) reports that 75% of children acquire their first episode of rotavirus infection before the age of one year, and severe infections occur in children aged 6 months-2 years (1,2).
Globally, rotavirus accounts for about 40% of acute diarrheal infections in children under the age of 5 years. In 2016, almost 258 million infections and 129,000 deaths were attributed to rotavirus infection, the biggest burden of which lay in low income countries. This could be because of limited access to health care, lack of available hydration therapy and a greater prevalence of comorbid conditions such as malnutrition in those countries (3–6).
In Uganda, diarrhea is still among the top ten causes of morbidity in children <5years. In 2019, rotavirus was the cause of almost half the acute diarrhea hospitalizations and an estimated 10,000 children died as a result (7). A recent study reported a prevalence of 16% among infants 3–24 months of age representing a reduction in prevalence(8).
After the global introduction of the rotavirus vaccine in 2006, rotavirus-associated mortality among children younger than 5 years decreased by 40% (3). Vaccinating infants further reduces rates of disease in older unvaccinated children and adults by reducing the circulating amount of rotavirus in the community.
On August 08th, 2023, four infants from a babies’ home were hospitalized at a general hospital in Kampala. They presented with a history of acute watery diarrhea, vomiting, general body weakness and signs of dehydration for more than 24 hours. Shortly after admission, one infant succumbed to their symptoms. Admitting clinicians ordered for laboratory tests and the results were positive for Rotavirus. The Ministry of Health (MoH) was notified of the suspected outbreak by the babies’ home administration. We determined the scope of the outbreak, confirmed the causative pathogen, identified possible exposures, and recommended evidence-based control and prevention measures.
Methods
Outbreak location
The outbreak took place at a babies’ home in Mpigi District. Mpigi District is located in Central Uganda. It has 8 subcounties, 59 parishes, and 481 villages. The babies’ home is located in Kiringente sub county and is nested in a children’s village. The home caters for 44 abandoned children from birth to 5 years (Figure 1). Majority (40/44) of the children at the babies’ home were vaccinated against rotavirus.
Case definition and finding
We defined a suspected case as acute onset of diarrhea plus ≥1 of the following: vomiting, fever, lethargy or loss of appetite in a child or staff member at the babies’ home from July 27─ Aug 20, 2023 OR A positive rotavirus RDT result in an asymptomatic child or staff member. A confirmed case was a suspected case with a positive Enzyme-linked immunoassay (ELISA) test for rotavirus A
To find cases we reviewed records of sick children from the babies’ home clinic. For each child enumerated from these records, we also conducted follow-up interviews with the clinicians and caretakers on the presentation of the symptoms. We also carried out rotavirus testing for all asymptomatic persons at the babies’ home using RDT kits. We then created a line-list of all cases who met the case definition capturing variables of dates of onset, demographic characteristics, symptoms, and vaccination status.
Descriptive epidemiology
We analyzed the line-list to characterize cases by their clinical presentation. We used attack rates to describe the distribution of cases by age group, vaccination status, and living area. We grouped the children in periods according to the time passed since they received the full 2-doses of vaccine. We defined three categories; <6 months since vaccination, 7-12, and >12 months since vaccination. We constructed an epi-curve to describe the cases by time.
Environmental assessment
We observed the layout of the babies’ home and examined the living spaces, the kitchen, food stores, living, and play areas. We observed the food handling and preparation practices. We interviewed the staff at the Babies’ Home on their hand hygiene as well as fecal matter handling and disposal practices.
Laboratory testing
We collected samples for laboratory testing from both clinical and non-clinical sources. We obtained samples of stool from the children and employees that were present during the outbreak period for testing using RDT kits. We tested 11 stool samples from symptomatic individuals using ELISA for rotavirus A (RVA) at the National Microbiology Reference Laboratory. We took non-clinical samples from the water sources at the babies’ home for bacterial culture.
Ethical considerations
This investigation was in response to a public health emergency and was therefore determined to be non-research by the office of the Center for Global Health, US Center for Disease Control and Prevention. The Ministry of Health gave the directive to investigate this outbreak. Administrative clearance to conduct the investigation was also obtained from the Mpigi District administration and the babies’ home administration.
Results
Descriptive epidemiology
We found 23 cases of rotavirus (6 confirmed; 17 suspected). Case fatality rate (CFR) for this outbreak was 4% with one death occurring. The average and median age of the child case-patients in this outbreak was 14 months, modal age was 10 months.
Children in the 13-18 months age group were most affected with attack rates of 86% followed by the 7-12 months ages group (83%). No cases were reported in the children above 2 years (Table 1).
Table 1: Attack rates by age group during an outbreak of rotavirus at a babies’ Home, Mpigi District, July─August 2023
| Age group | No. of Cases | Population | AR (%) |
| 0-6 months | 3 | 6 | 50 |
| 7-12 months | 5 | 6 | 83 |
| 13-18 months | 6 | 7 | 86 |
| 19-24 months | 7 | 10 | 70 |
| 2-5 years | 0 | 15 | 0 |
| ≥ 18 years | 2 | 59 | 3.4 |
Acute watery diarrhea was reported for 19/23 of case-patients, followed by vomiting (18/23), general body weakness (GBW) (11/23), and loss of appetite (8/23). Thirteen percent of the cases did not experience any symptoms (Figure 2).
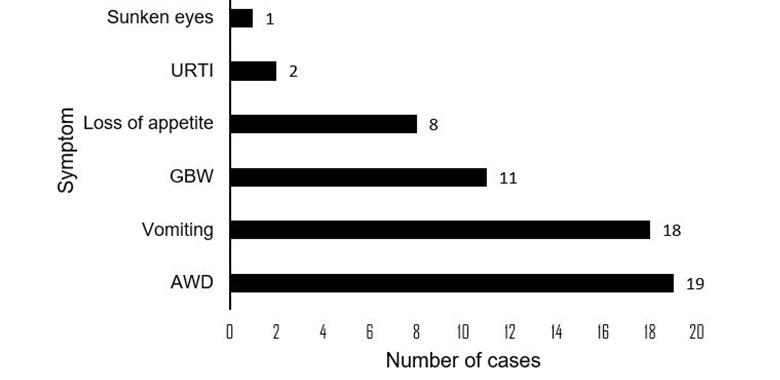
Figure 2: Frequency of rotavirus symptoms among affected children at a babies’ home in Mpigi district, 2023
Ninety-five percent of the child case-patients were vaccinated. The only unvaccinated child was not yet eligible for vaccination on account of their age. Children that received the vaccine 7-12 months before the outbreak had highest attack rates compared to other categories (Table 2).
Table 2: Time in months since vaccination vs Attack rates and Risk Ratios
| Time since vaccination (months) | Infected | Total | AR (%) | RR | CI limits |
| <6 | 4 | 7 | 57 | Ref | |
| 7-12 | 6 | 6 | 100 | 1.75 | 0.92-3.32 |
| >12 | 10 | 26 | 38 | 0.67 | 0.78-3.91 |
The primary case fell ill on August 03rd, days after, multiple cases formed a first generation of cases (from August 5th-8th) (Figure 3). A second generation of cases were recorded from August 10-14. The single case that died did so on August 10th, and this spurred the notification of MoH on August 11th. More cases that may have been the third generation of cases appeared on August 17-18th, 2023.
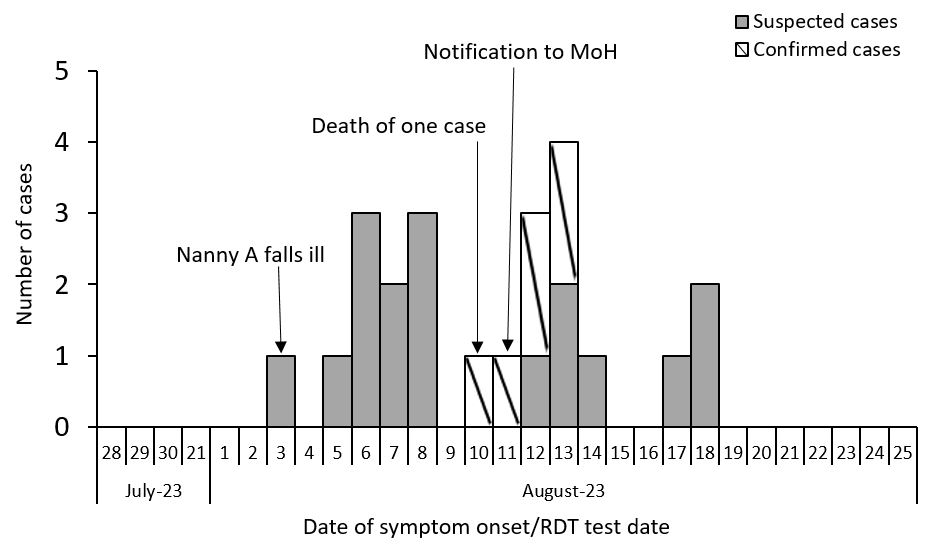
Laboratory findings
From the 103 babies’ home residents and employees, 74 were tested for RV by RDT and 18 (24%) tested positive (Table 3). From the 11 stool samples collected from symptomatic persons, 6 (55%) tested positive on ELISA. No organism known to cause gastrointestinal infections was found by microbiology culture from both the water and stool samples.
Table 3: Laboratory results for the rotavirus outbreak at a babies’ home in Mpigi District, July-August 2023
| Test run | Samples collected | Positive n (%) | Negative n (%) |
| RDT | 74 | 18 (24) | 56 (76) |
| ELISA | 11 | 6 (55) | 5 (45) |
| Culture (stool) | 11 | 0 (0) | 11(100) |
| Culture (water) | 7 | 0 (0) | 7 (100) |
Environmental assessments
Babies’ home layout: The babies’ home is housed on two floors, most of the children live on the ground floor according to their age groups (Figure 4). Within age groups, they are separated into family units of four and are assigned to a specific caretaker. Mixing between children in the different age groups is not common. The children had different schedules based on their age and developmental needs. The common areas at the babies’ home were the children’s play area and the early childhood development playroom. There was minimal interaction between the older children (>2 years) and other age groups.
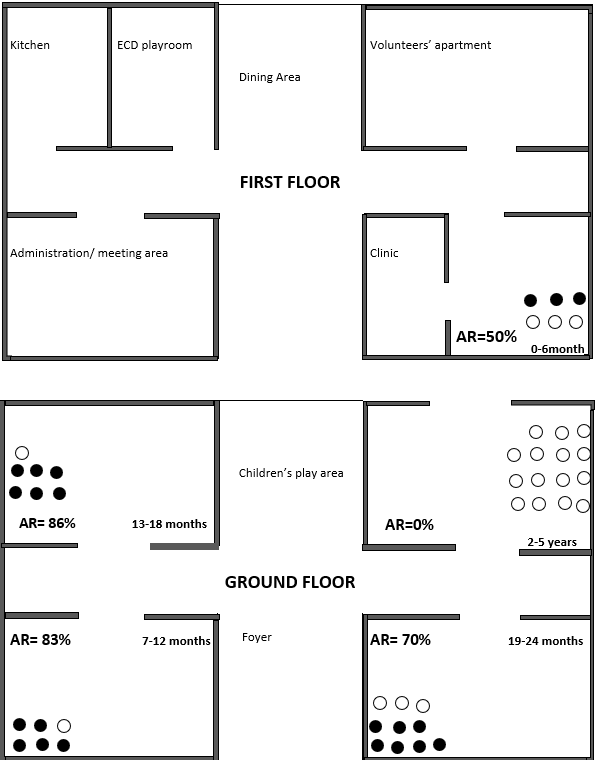
![]() Figure 4: Attack rates by living area at the babies’ home in Mpigi District
Figure 4: Attack rates by living area at the babies’ home in Mpigi District
We however noted that the clinic was a high traffic area and that the cribs were the children were placed were not disinfected between case patients.
Food preparation and handling: The babies’ home has two chefs who prepare food for both the children and the staff. Dry rations were purchased in bulk and stored in a pantry adjacent to the kitchen, fresh food was delivered on preset days according to the children’s menu and also stored appropriately. The children had a separate menu from the adults, and menus varied for the different child age groups as well. Food prepared for the children was served into containers and placed on a counter for pick up.
No food was left over for the next meal as menus between meals varied. The cooks reported that nothing changed in the food delivery and preparation process the week leading up to the outbreak. The same suppliers were used and food was prepared and served in the same way that food was normally prepared.
Water sources: Water used at the babies’ home was pumped from a deep well located about 1.5 kilometers away and stored in plastic above ground water tanks. The same water was pumped and used at the children’s village as well and there were no reports of related illness. There was no evidence of deviation from the norm.
Fecal matter and vomitus handling: Children in the 0-12 months age group were kept in diapers. Diaper changes were done at the start of each day, and whenever necessary thereafter and gloves were worn during each diaper change. Children in the 13-24 months age group were potty trained. Each child had their own potty, no sharing was reported or witnessed. Caretakers reported that when children were woken up, they had “potty-time” session, after which the child is cleaned and the contents of the potty taken to a toilet outside the living area for disposal. We observed one such potty disposal session and noted that while disposal happened outside the living area, the caretaker had to walk back to the living area to clean the potty. Potties were rinsed with soapy water in a sink within the living area.
We found that only 39% (9/23) used disposable gloves and washed the spill area with detergent after cleaning (Table 4). Only 17% (4/23) reported washing their hands after the spills cleaning process and kept other children away from the spill area. Only 39% (9/23) washed their hands before and after feeding the children, before handling the children’s toys and after washing the children’s’ potties.
Table 4: Procedure for cleaning of vomitus and diarrheal spills and handwashing
| Action | n | % |
| Put on disposable gloves | 9 | 39 |
| Wash the surface with a soap/detergent | 9 | 39 |
| Keep other babies away from soiled area | 4 | 17 |
| Wash hands after the cleaning procedure | 4 | 17 |
| Self-reported handwashing times | ||
| After feeding the babies | 9 | 39 |
| Before feeding the babies | 9 | 39 |
| Before touching utensils | 6 | 26 |
| Before changing baby’s diapers | 3 | 13 |
| Before touching toys | 9 | 39 |
| After washing potties | 9 | 39 |
None of the staff reported washing their hands between feeding the children or when visibly soiled. At the time of the outbreak, staff reported a shortage of gloves.
Discussion
In this study, we carried out an epidemiological investigation on the outbreak of rotavirus in a babies’ home in Mpigi District. We confirmed that Rotavirus A was the causative pathogen and both children and adult staff at the babies’ home were affected. All the affected children were under 2 years but those aged between 13-18 months were most affected (AR=86%). One death occurred in an infant who died from rotavirus-induced dehydration (CFR= 4%). Majority (95%) of the child case patients affected by the outbreak were vaccinated. The outbreak was linked to poor hygiene practices among the care givers.
In this study, we confirmed that the outbreak at the babies’ home was caused by Rotavirus A. Laboratory results showed that six out of eleven samples from symptomatic children tested positive for rotavirus A using ELISA. Similar to our findings, studies of rotavirus disease in children under the age of five years in central Uganda and Nigeria found that the most prevalent genotype of rotaviruses detected were group A rotaviruses (9,10).
In this study we found that children affected were under 2 years but those aged between 13-18 months had the highest attack rates. Similar to our findings, a study on children under the age of five years hospitalized with acute gastroenteritis reported that a majority were <24 months of age (11). This could be because older children are more resistant to rotavirus on account of their previous exposures to the pathogen which confers against reinfection and severe diarrhea. Two of the case patients in this outbreak were adults. Rotavirus infection in adults is not uncommon, as supported by research that found that nearly all adults have antibodies to rotavirus but also found that they were still susceptible to infection (12).
One death attributed to rotavirus-induced dehydration occurred during this outbreak. This is similar to the findings in an outbreak in subacute inpatient care facility where a case patient <2 years died due to rotavirus-induced dehydration (13). While the case patient in that outbreak was not vaccinated unlike the case patient in our outbreak, rotavirus diarrhea can quickly lead to serious dehydration without quick intervention.
We found that 95% of the child case patient affected by the outbreak were vaccinated, all having received the Rotarix ® vaccine at least six weeks prior to the outbreak. Rotarix (®) is a live attenuated vaccine and has been associated with causing acute gastroenteritis in vaccine recipients. This outbreak in a vaccinated closed population is contrary to other outbreaks in similar populations but largely unvaccinated (13,14). This could be because rotavirus is easily spread in settings where many children are together, such as day care, or other child care facilities.
In this study we found that 83% of the case-patients had symptoms ranging from acute watery diarrhea, vomiting, and general body weakness among others. Rotavirus is a known cause of severe dehydrating diarrhea. This could be possible reason for the single fatality in this study that reportedly occurred due to severe dehydration from loss of fluid and electrolytes in the watery stool (15).
During the environmental assessments, we noted that the handwashing station at the home entrance was not utilized. Thus staff were likely to report and likely handle the children without washing their hands.
We also evaluated the fecal matter handling processes for the children and noted that more than 20% of the caregivers were not knowledgeable on the proper procedure of cleaning up vomitus and diarrhea. And since RV is found in human feces and can be transmitted through the fecal-oral route, washing of hands with soap and water before handling a child halts its transmission (6).
Several studies have shown that caregiver’s handwashing with soap is linked with a low prevalence of diarrheal diseases among children (8,9). The same assessments revealed a possible source of exposure from the fecal management process. Children would defecate in potties and caregivers would dispose of the feces from the potty into the toilet, then clean the potty for the next use.
The training given to staff prior to starting employment were lacking. Absence of essential IPC training increases the risk of exposure to infectious agents for the persons receiving care as the caregivers may not have proper knowledge and understanding of correct disease prevention processes and procedures.
Study limitations
Given that the key informants are staff at the babies’ home, it is likely that the responses to the questions including the staff health prior to the outbreak were not truthful; making it impossible to ascertain the true source of infection.
We were unable to perform genomic sequencing on the clinical samples we had taken hence unable to tell if the RVA was a wildtype or as a result of vaccination, something which would further facilitate in shaping the recommendations.
Public health actions taken
Immediately after the outbreak was detected, we started sensitization of the health workers and staff at the babies’ home. With the help of the Mpigi district health team, we disinfected the babies’ home distributing of hand washing facilities at entry points.
Conclusion
We concluded that the outbreak that occurred among children and staff at the babies’ home was caused by Rotavirus A. The outbreak was linked to poor hygiene practices among the care givers.
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ contribution
All authors contributed to the write-up and review of the bulletin. DA wrote the drafts of the bulletin and revised the bulletin for substantial intellectual content. DA, PA, BK, and BG participated in the investigation of rotavirus in Mpigi district and reviewed the bulletin for substantial intellectual content. SG and DNG were involved in the review of the bulletin for substantial intellectual content. BK and ARA participated in the supervision of field data collection and reviewed the draft manuscript for substantial intellectual content. All the authors read and approved the final version of the bulletin.
Acknowledgements
The authors thank the staff of the Uganda Public Health Fellowship Program for the technical support and guidance offered during this study. The authors also extend their appreciation to Mpigi District team for the support they offered during the investigation. We appreciate the National Microbiology Reference Laboratory for testing all samples and the timely release of laboratory results. Finally, we thank the US-CDC for supporting the activities of the Uganda Public Health Fellowship Program.
Copyright and licensing
All materials in the Uganda Public Health Bulletin are in the public domain and may be used and reprinted without permission; citation as to source; however, is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
Reference
- Rotavirus [Internet]. [cited 2023 Aug 29]. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/rotavirus
- Transmission of Rotavirus | CDC [Internet]. 2022 [cited 2023 Aug 30]. Available from: https://www.cdc.gov/rotavirus/about/transmission.html
- Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality from Diarrhea. J Infect Dis. 2017 Jun 1;215(11):1666–72.
- Du Y, Chen C, Zhang X, Yan D, Jiang D, Liu X, et al. Global burden and trends of rotavirus infection-associated deaths from 1990 to 2019: an observational trend study. Virol J. 2022 Oct 20;19(1):166.
- Ramig RF. Pathogenesis of Intestinal and Systemic Rotavirus Infection. J Virol. 2004 Oct;78(19):10213–20.
- Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, et al. Rotavirus infection. Nat Rev Dis Primer. 2017 Nov 9;3:17083.
- Patrick L. Uganda rolls out rotavirus vaccine into the routine immunization schedule [Internet]. Ministry of Health | Government of Uganda. 2019 [cited 2024 Jan 29]. Available from: https://www.health.go.ug/2019/12/02/uganda-rolls-out-rotavirus-vaccine-into-the-routine-immunization-schedule/
- Nakawesi JS, Wobudeya E, Ndeezi G, Mworozi EA, Tumwine JK. Prevalence and factors associated with rotavirus infection among children admitted with acute diarrhea in Uganda. BMC Pediatr. 2010 Sep 24;10:69.
- Bwogi J, Malamba S, Kigozi B, Namuwulya P, Tushabe P, Kiguli S, et al. The epidemiology of rotavirus disease in under-five-year-old children hospitalized with acute diarrhea in central Uganda, 2012-2013. Arch Virol. 2016;161:999–1003.
- Mado SM, Giwa FJ, Abdullahi SM, Alfa AM, Yaqub Y, Usman Y, et al. Prevalence and Characteristics of Rotavirus Acute Gastroenteritis among Under-five Children in Ahmadu Bello University Teaching Hospital, Zaria, Nigeria. Ann Afr Med. 2022;21(3):283–7.
- Nguyen TV, Le Van P, Le Huy C, Weintraub A. Diarrhea Caused by Rotavirus in Children Less than 5 Years of Age in Hanoi, Vietnam. J Clin Microbiol. 2004 Dec;42(12):5745–50.
12.Anderson EJ, Weber SG. Rotavirus infection in adults. Lancet Infect Dis. 2004 Feb 1;4(2):91–9.
- Burke RM, Tate JE, Barin N, Bock C, Bowen MD, Chang D, et al. Three Rotavirus Outbreaks in the Postvaccine Era — California, 2017. Morb Mortal Wkly Rep. 2018 Apr 27;67(16):470–2.
- Burke RM, Tate JE, Han GS, Quenelle R, Gautam R, Wadford DA, et al. Rotavirus vaccination coverage during a rotavirus outbreak resulting in a fatality at a subacute care facility. J Pediatr Infect Dis Soc. 2020 Jul 13;9(3):287–92.
- Rotavirus Infection in Children – Health Encyclopedia – University of Rochester Medical Center [Internet]. [cited 2023 Aug 20]. Available from: https://www.urmc.rochester.edu/encyclopedia/content.aspx?Contenttypeid=90&contentid=P02540


Comments are closed.