Investigation of a Bacterial Meningitis Cluster in a Refugee Settlement, Obongi District, Uganda, March, 2023
Authors: Brian Agaba1*, Rebecca Akunzirwe1, Leah Naluwagga1, Paul Okello1, Daniel Kadobera1, Richard Migisha1, Alex Riolexus Ario1 Institutional affiliations: 1Uganda Public Health Fellowship Program-Uganda National Institute of Public Health, Kampala, Uganda; *Correspondence: Tel: +256774125554, Email: agababrian@uniph.go.ug
Summary
Background: On 6 March 2023, the Uganda Central Public Health Laboratory isolated Neisseria Meningitidis serogroup C from a cerebral spinal fluid sample from Obongi District. This sample was one of many from patients that were presenting with fever, convulsions, and altered consciousness. We investigated to determine the scope and magnitude of the meningitis cluster/ outbreak, identify risk factors of contracting meningitis, and inform control/prevention measures.
Methods: We defined a suspected case as a resident of Palorinya Refugee Settlement with sudden onset of fever (>37.5 °C), and any one of neck stiffness, convulsions, altered consciousness, coma or bulging fontanelle in infants from 1 December 2022 to 1 May 2023. A probable case was any suspected case with macroscopic aspect of cerebrospinal fluid (CSF): turbid, cloudy or purulent; or with a CSF leukocyte count >10 cells/mm3 or with bacteria identified by Gram stain in CSF; or positive antigen detection (for example, by latex agglutination testing) in CSF. In infants, a probable case was CSF leucocyte count >100 cells/mm3; or CSF leucocyte count 10–100 cells/mm3 and either an elevated protein (>100 mg/dl) or decreased glucose (<40 mg/dl) level. A Confirmed case was any suspected/probable case that was laboratory confirmed by culturing or identifying (polymerase chain reaction) a bacterial pathogen (Neisseria meningitidis, Streptococcus pneumoniae, Haemophilus influenzae type b) in the CSF or blood. We reviewed medical records, conducted active case finding, and conducted key informant interviews in the affected communities to identify cases and factors associated with contracting meningitis. We analysed case data by person, place, and time.
Results: Between 22 December 2022 and 1 May 2023, 25 cases with 2 deaths of bacterial meningitis occurred in Palorinya Refugee Settlement, Obongi District. Of these, 4 were laboratory confirmed with Neisseria meningitidis serogroup C, 6 were probable cases, and 15 were suspected cases. Most (76%) of case-patients were <18 years with a median age of 12 years (range 1-66 years). None of the case-patients was vaccinated against Neisseria meningitidis serogroup C. Each case-patient was from a different household and there was no epidemiological link between any of the cases. Conclusion: This meningococcal meningitis cluster caused by Neisseria meningitidis serogroup C occurred among non-vaccinated persons mostly aged <18 years in Palorinya Refugee Settlement. We recommended vaccination of at-risk persons. As a result of this investigation, at the national level, a technical working group was setup to monitor the cluster of cases. At district level, surveillance activities were continued until 2 weeks after the last case.
Background
Bacterial meningitis is a clinical syndrome characterized by inflammation of the meninges that cover the brain and spinal cord. Up to 95% of patients have at least 2 of the 4 following symptoms: fever, headache, stiff neck, or altered mental status/convulsions [1]. Bacterial meningitis is commonly caused by Streptococcus pneumoniae, Haemophilus influenza, and Neisseria meningitidis.
Most outbreaks are now caused by Neisseria meningitidis [2, 3]. With humans being the reservoir, bacterial meningitis is transmitted through direct contact from person to person through droplets of respiratory or throat secretions from infected people. The incubation period ranges from 2-10 days with an average of 3-4 days [4]. Case fatality rate ranges from 5-15% with up to 20% of survivors suffering long term complications such as hearing loss, seizures, limb weakness, difficulties with vision, speech, language, and memory [4].
Uganda lies in the meningitis belt of Sub-Saharan Africa and experiences frequent outbreaks of bacterial meningitis. An analysis of surveillance data between 2015 and 2018 showed that the incidence of bacterial meningitis in Uganda was on the increase [5]. In Uganda, the most susceptible regions include West Nile, Bunyoro, Acholi, Lango, Teso, and Karamoja regions [5].
Meningitis outbreak response in the meningitis belt of sub-Saharan Africa encompasses strengthening surveillance for early outbreak investigation, strengthening case management, reactive vaccination of susceptible persons early on in the outbreak and routine mass vaccination campaigns in areas at greatest risk [6]. As per the Uganda national technical guidelines for Integrated Disease Surveillance and Response (IDSR), bacterial meningitis is one of those diseases that must be reported immediately [7].
These guidelines stipulate two thresholds for public health action with regards to bacterial meningitis. The alert threshold is reached when there are 2 cases per week in an area with less than 30,000 inhabitants or an attack rate of 5 cases per 100,000 inhabitants per week in an area with a population of 30,000 to 100,000 inhabitants. The epidemic threshold is reached when there are 10 suspected cases per 30,000 – 100,000 inhabitants per week or 5 suspected cases in one week in an area with less than 30,000 inhabitants [7]. Despite repeated outbreaks, availability of national and international guidelines, efforts to control bacterial meningitis remain suboptimal for many of the countries in the meningitis belt leading to frequent outbreaks [8].
On 6 March 2023, the Uganda Central Public Health Laboratory isolated Neisseria meningitidis from a cerebral spinal fluid sample from Obongi District, Uganda. This sample was one of many from patients that were presenting with fever, convulsions, and altered consciousness. We investigated to determine the occurrence, scope and magnitude of a meningitis outbreak, identify risk factors of contracting meningitis, and inform control/prevention measures.
Methods
Study setting
Obongi District is located in West Nile, one of the regions of Uganda located within the extended meningitis belt of Sub-Saharan Africa. It is bordered by Moyo District to the north, Adjumani District to the east, Yumbe District to the west, and Madi Okollo District to the south (Figure 1). The district has a population of 173,325 with 122,000 (70%) being refugees from South Sudan. These refugees have occupied Palorinya Refugee Settlement since 2016. This settlement spans an area of 37.58 Km2 and is comprised of 31 villages across two Sub-counties of Obongi District. Palorinya Refugee Settlement is located about 30 kilometres from the South Sudan boarder. There is frequent movement of people between the settlement and South Sudan. At the time of the outbreak, there are no ongoing vaccination efforts of refugees against meningitis at entry or while in the settlement. Obongi District last carried out a vaccination campaign in 2017 against Neisseria Meningitidis serogroup A. The District has never vaccinated people against Neisseria Meningitidis serogroup C.
Case definition and case finding
We defined a suspected case as a resident of Palorinya Refugee Settlement with sudden onset of fever (>37.5 °C), and any one of neck stiffness, convulsions, altered consciousness, coma or bulging fontanelle in infants from 1 December 2022 to 1 May 2023 [7]. A probable case was any suspected case with macroscopic aspect of cerebrospinal fluid (CSF) turbid, cloudy or purulent; or with a CSF leukocyte count >10 cells/mm3 or with bacteria identified by Gram stain in CSF; or positive antigen detection (for example, by latex agglutination testing) in CSF. In infants, a probable case was CSF leucocyte count >100 cells/mm3; or CSF leucocyte count 10–100 cells/mm3 and either an elevated protein (>100 mg/dl) or decreased glucose (<40 mg/dl) level. A Confirmed case was any suspected/probable case that is laboratory confirmed by culturing or identifying (polymerase chain reaction) a bacterial pathogen (Neisseria meningitidis, Streptococcus pneumoniae, Haemophilus influenzae type b) in the CSF or blood [7].
We conducted active case search in the community in Palorinya Refugee Settlement and neighboring villages with assistance from community health workers and the district surveillance focal person. We further reviewed the medical records of the health facilities serving the affected villages to identify more cases.
We interviewed the suspected case-patients to identify factors likely associated with contracting the infection. We explored these factors: travel to South Sudan, vaccination against meningitis, and household size.
Information on symptoms and signs, date of onset, date of presentation/admission to hospital, duration of illness, drugs, laboratory results, and medical complications was obtained by interviewing the case-patients and reviewing their medical records.
Descriptive epidemiology
We calculated proportions to describe the distribution of cases by age, sex, and symptoms. We also described case-patients by time of onset of symptoms using an epidemiological curve and calculated attack rates to describe the distribution of cases by place (village). We calculated time from symptom onset to presentation at health facility. A graph of attack rates per week was plotted to determine whether this increase in meningitis cases had reached the epidemic thresh hold.
Factors likely associated with contracting meningitis infection
We conducted key informant interviews with the District health officer, Palorinya Refugee Settlement leadership, District surveillance focal person, health facility leaders, and the community health workers of the affected villages to identify possible sources and factors associated with contracting bacterial meningitis.
Ethical considerations
This investigation was in response to a public health emergency and was therefore determined to be non-research. The Ministry of Health gave a directive to investigate this possible outbreak. The authors sought permission to conduct the investigation from District health authorities of Obongi District, administrators of Palorinya Refugee Settlement, and the health facilities. The authors sought verbal informed consent from the respondents who were at least 18 years old as well as those that were below 18 years of age and emancipated. The authors also sought assent from children below 18 years of age who were not emancipated and informed verbal consent from their parents or guardians.
This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.§§See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C.
- 241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Results
Descriptive epidemiology
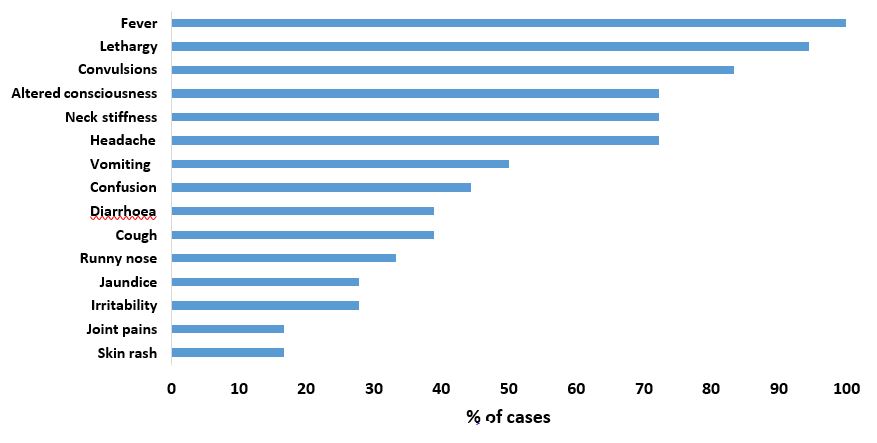
We line listed a total of 25 cases of bacterial meningitis. Of these, 4 were confirmed cases, 6 were probable cases, and 15 were suspected cases. The case fatality rate was 8% (2/25). The median age of the case-patients was 12 years (range 1 to 66). Most (76%) of the case-patients were <18 years. Case-patients presented with fever (100%), lethargy (94%), convulsions (83%), headache (72%), neck stiffness (72%), altered consciousness (72%), vomiting (50%), confusion (44%), diarrhea (39%), cough (39%) and runny nose (33%) (Figure 2). In this cluster, 2/25 case-patients reported complications related to meningitis (hearing loss (1/25) and limb weakness (1/25)).
The overall attack rate (AR) for bacterial meningitis in Palorinya Refugee Settlement was 21/100,000 population. Persons aged 12-17 years were the most affected (AR 43/100,000) followed by 1-4 years (AR 33/100,000), >59 years (AR 20/100,000), 5-11years (AR 15/100,000), 18-59 years (AR 10/100,000). Males (AR 30/100,000) were more affected than females (AR 12/100,000). All weeks in which meningitis cases were reported had attack rates below the epidemic threshold of 10 cases per 100,000.
The median household size was 7 (range 1 to 10) people with 3 (range 1 to 6) people sharing a bedroom. None of the case-patients had travelled out of the refugee settlement in the six months prior to illness. All case-patients received health care from government health facilities. The majority (83%) of case-patients sought medical care within 24 hours of symptom onset. All case-patients were treated with Ceftriaxone antibiotic.
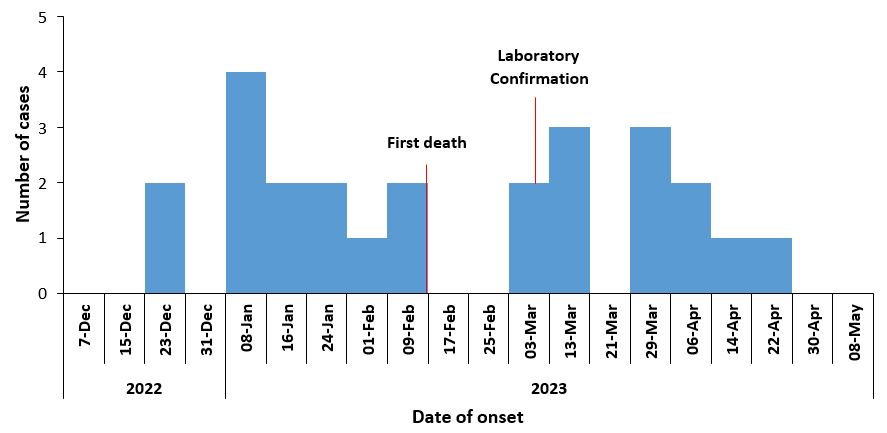
This cluster of bacterial meningitis started on 22 December 2022 and ended on 1 May 2023.The first case of meningitis occurred on 22 December 2022 and laboratory confirmation of meningitis was on 6 March 2023 (Figure 3).
As of May 1, 2023, nine villages had reported cases of Bacterial meningitis. Seven of the villages were in Palorinya Refugee Settlement while two of the villages were neighboring the settlement. The affected villages were scattered all over the refugee settlement. Some affected villages were more than 20 Kilometers apart. Luakoke (AR=79/100,000) and Keguru (AR=64/100,000) were the most affected villages (Table 1).
Table 1: Attack rate by village during the Bacterial meningitis cluster, Obongi District, Uganda, December 2022 – May 2023
| Village | Cases | Population | Attack rate/100,000 |
| Luakoke | 2 | 2,539 | 79 |
| Belameling | 5 | 7,737 | 65 |
| Keguru | 6 | 9,378 | 64 |
| Luru | 3 | 7,649 | 39 |
| Idiwa | 4 | 12,354 | 32 |
| Dongo | 1 | 4,720 | 21 |
| Pasu | 1 | 9,337 | 11 |
| Kali | 2 | NP | |
| Umbechi | 1 | NP |
NP: Population data not available, AR: Attack rate
Factors likely associated with contracting meningitis infection
Authorities reported frequent to and from movement of persons from South Sudan and suspected that the cases could be associated to the movement. Although authorities suspected that this cluster could have been imported from South Sudan, there was no evidence to point to that, they had not contacted their counterparts in South Sudan to find out whether there was an ongoing meningitis outbreak.
None of the case-patients was vaccinated against Neisseria Meningitidis serogroup C. Only 2/25 case-patients reported history of vaccination. This was in 2017 with a vaccine against Neisseria Meningitidis serogroup A.
Discussion
This cluster of meningitis caused by Neisseria meningitidis serogroup C was confined to Palorinya Refugee Settlement and neighbouring villages. Although the cluster started on 22 December 2022, the ministry of health was alerted ten weeks later on 6 March 2023. We line listed 25 cases with a case fatality rate of 8% and a complication rate of 8%. The cases were sporadically distributed across an area of 20 Km2 with no epidemiological link between them. No household reported more than one case. Case- patients had good health seeking behaviour and were all appropriately treated with effective antibiotics. There was frequent in and out movement of persons between South Sudan and the Palorinya Refugee Settlement. There was no vaccination campaign against bacterial meningitis in the district in the last 5 years.
Although the first case of meningitis presented to the health facility on 22 December 2022, the Ministry of Health was only alerted 10 weeks later. According to the Uganda National Technical guidelines on Integrated Disease Surveillance and Response [7], bacterial meningitis is one of the diseases requiring immediate reporting. Surveillance forms the back bone of bacterial meningitis control. Official declaration of a bacterial meningitis outbreak, resource mobilisation, effective case management and reactive mass vaccination campaigns all depend on an efficient surveillance system [9, 10]. Our investigation revealed a gap in surveillance that needs to be addressed to better protect the at-risk communities against bacterial meningitis.
This cluster of meningitis was caused by Neisseria meningitidis serogroup C. Studies have shown that serogroup C is becoming a major cause of meningococcal meningitis in the meningitis belt of Sub-Saharan Africa[11, 12]. This is because there have been minimal vaccination campaigns against Serogroup C leaving at risk populations susceptible to this serogroup. As of 1 May 2023, there was no documented vaccination campaign against serogroup C in Uganda. In contrast, other causes of bacterial meningitis have been targeted through routine immunisation of children with Haemophilus influenzae and pneumococcal vaccines. In 2017, there was vaccination against Neisseria meningitidis serogroup A in Obongi and 38 other districts in Uganda. This could explain why the other causative organisms for bacterial meningitis are declining while serogroup C is increasing. Mass vaccination campaigns have shown efficacy in the meningitis belt of Africa [4, 13].
The case fatality rate (8%) and the complication rate (8%) of bacterial meningitis in this outbreak was low. Studies show that 5-15% of bacterial meningitis cases die while 20% of survivors suffer long term complications such as hearing loss, seizures, limb weakness, difficulties with vision, speech, language and memory [4, 14]. In this outbreak, 83% of case patients received medical care within 24 hours of symptom onset and were all treated appropriately with an effective antibiotic. This could explain the low case fatality and complication rates seen in this outbreak. Without treatment, case fatality rates for bacterial meningitis increase up to 50% [15].
Our investigation failed to establish an epidemiological link between any of the case- patients. None of the affected households had more than one case. Studies show that most cases of bacterial meningitis are spread from asymptomatic carriers [16].
Literature on the epidemiology of bacterial meningitis shows that 10% to 20% of the population carries Neisseria meningitidis in their throat at any given time [15]. However, less than 1% of persons colonised with Neisseria meningitidis progress to invasive disease [17-19]. It is likely that these case-patients acquired the infection from asymptomatic carriers.
Study limitations
This investigation was started 10 weeks after the first case of Bacterial meningitis presented to hospital. As a result, our findings could be affected by recall bias. Case- patients were reluctant to discuss travel in and outside the refugee settlement and felt this could jeopardize their refugee status. This could have led to social desirability bias.
Conclusion
This cluster of meningococcal meningitis caused by Neisseria meningitidis serogroup C did not reach the epidemic thresh hold stipulated in the IDSR guidelines. None of the affected persons was vaccinated against Neisseria meningitidis serogroup C.
We recommend strengthening meningitis surveillance through cross border collaboration between Uganda and South Sudan, training of health workers in case detection and reporting. Mass vaccination of at-risk persons with vaccines targeting common Neisseria meningitidis serogroups (A, B, C, W, Y) could reduce the magnitude, case fatality, and complication rates of outbreaks.
Public health actions
The results from this investigation were shared with the Ministry of Health National Task Force, Palorinya Refuge Settlement leadership, and Obongi District health authorities.
As a result, at the national level, a technical working group was setup to monitor the cluster/outbreak and establish a working relationship with South Sudan health authorities to share information. At district level, surveillance activities were continued until 2 weeks after the last case. We supported the district to make daily situation reports of the outbreak.
Conflict of interest
The authors declare that they had no conflict of interest.
Authors contribution
BA, RA, LN, PO conducted the investigation and contributed to report writing. DK, RM, ARA critically reviewed the report for intellectual content.
Acknowledgments
The authors would like to thank the Ministry of Health, Obongi District local government, and Palorinya Refugee Settlement leadership for their permission and support in conducting this investigation.
Copyright and licensing
All materials in the Uganda Public Health Bulletin is in the public domain and may be used and reprinted without permission; citation as to source; however, is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- van de Beek, D., et al., Clinical Features and Prognostic Factors in Adults with Bacterial Meningitis. New England Journal of Medicine, 2004. 351(18): p. 1849-
- Quagliarello, V. and W.M. Scheld, Bacterial Meningitis: Pathogenesis, Pathophysiology, and New England Journal of Medicine, 1992. 327(12): p. 864-872.
- Brouwer, M.C., A.R. Tunkel, and D.v.d. Beek, Epidemiology, Diagnosis, and Antimicrobial Treatment of Acute Bacterial Meningitis. Clinical Microbiology Reviews, 23(3): p. 467-492.
- Meningitis. WHO fact sheet [Fact sheet] 2021 28 September 2021; Available from: https://www.who.int/news-room/fact-sheets/detail/meningitis.
- Gonahasa, N., et al., Incidence and Spatial Distribution of Bacterial Meningitis, Uganda, 2015-2018. 2023.
- Stephens, S., Protecting the herd: the remarkable effectiveness of the bacterial meningitis polysaccharide-protein conjugate vaccines in altering transmission dynamics. Trans Am Clin Climatol Assoc, 2011. 122: p. 115-23.
- Uganda, M.o.H., National Technical Guidelines for Integrated Disease Surveillance and Response, M.o. Health, Editor. 2021, Ministry of Health: p. 485.
- Greenwood, B., S. Sow, and M.-P. Preziosi, Defeating meningitis by 2030 – an ambitious target. Transactions of The Royal Society of Tropical Medicine and Hygiene, 115(10): p. 1099-1101.
- Preventing and controlling meningitis outbreaks. 2023 [cited 2023 20/8/23]; Available from: https://www.who.int/activities/preventing-and-controlling- meningitis-outbreaks.
- Harrison, L.H., et al., The Global Meningococcal Initiative: recommendations for reducing the global burden of meningococcal disease. Vaccine, 2011. 29(18): p. 3363-3371.
- Godjedo, T.P.M., et al., Epidemiological Characteristics of Meningococcal Meningitis (2016 to 2018) Four Years after the Introduction of Serogroup A Meningococcal Conjugate Vaccine in Benin. Advances in Public Health, 2020. 2020: 1932940.
- Peterson, E., et al., Meningococcal serogroups and surveillance: a systematic review and survey. Journal of global health, 2019. 9(1).
- Pelton, I., The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. Journal of Adolescent Health, 2016. 59(2): p. S3-S11.
- Wang, B., et al., Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine, 2019. 37(21): 2768-2782.
- Meningococcal Meningitis. 2023 2023 [cited 2023 13 August 2023]; Available from: https://www.afro.who.int/health-topics/meningococcal-meningitis.
- Pavlopoulou, I., et al., Carriage of Neisseria meningitidis by Greek children: risk factors and strain characteristics. Clinical microbiology and infection, 2004. 10(2): 137-142.
- Caugant, D.A., et al., Transmission of Neisseria meningitidis among asymptomatic military recruits and antibody analysis. Epidemiol Infect, 1992. 109(2): 241-53.
- Le Saux, N., et al., Carriage of Neisseria species in communities with different rates of meningococcal disease. Can J Infect Dis, 3(2): p. 60-4.
- He, , et al., Neisseria meningitidis carriage and risk factors among teenagers in Suizhou city in China. Epidemiol Infect, 2020. 148: p. e227.


Comments are closed.