Trends and spatial distribution of neonatal sepsis, Uganda, 2016-2020
Authors: Stella M. Migamba1*, Esther Kisaakye1, Miriam Nakanwagi1, Allan Komakech1, Petranilla Nakamya1, Benon Kwesiga1,2, Lilian Bulage1,2, Robert Mutumba3, Deogratius Migadde3, Alex R. Ario1,2 Institutional Affiliations; 1Uganda Public Health Fellowship Program, Kampala, Uganda, 2Uganda National Institute of Public Health, Kampala, Uganda, 3Ministry of Health, Reproductive and Infant Health Department, Kampala, Uganda *Correspondence: Email: smigamba@musph.ac.ug, Tel: +256-774-662-488
Summary
Background: Neonatal sepsis is the third-leading cause of neonatal deaths in Uganda. The infection can be acquired perinatally (early-onset sepsis (EOS) occurring within seven days postpartum) or nosocomially or in the community (late-onset sepsis (LOS) occurring 8-28 days postpartum). We described trends and spatial distribution of neonatal sepsis in Uganda, 2016-2020.
Methods: We analysed routinely-reported surveillance data on in-patient neonatal sepsis from the District Health Information Software version 2 (DHIS2) during 2016-2020. We analysed sepsis distribution by district and calculated incidence rates for EOS, LOS, and total sepsis at district, regional, and national levels as cases per 1,000 live-births (LB), as well as total sepsis incidence by health facility level. We determined significance of national and regional trends using logistic regression and the corresponding odds ratios. We demonstrated the spatial distribution using choropleth maps.
Results: During 2016-2020, 95,983 neonatal sepsis cases were reported and of these, 71,262 (74.2%) were EOS. Overall incidence of neonatal sepsis was 17.4/1,000 live-births. Nationally, incidence of sepsis was generally highest at the Regional Referral Hospital (RRH) level (68/1000 live-births) and lowest at the Health Centre II level (1.3/1000 live-births). The change in total sepsis during 2016 to 2020 was not statistically significant (P=0.133). However, EOS increased from 11.7 to 13.4 cases/ 1000 LB, with an average yearly increase of 3% (p<0.001) and late-onset sepsis declined from 5.7 to 4.3 cases/1000 LB with an average yearly decrease of 7% (p<0.001). Regionally, early onset sepsis as well as total sepsis increased in Central (15.5 to 23.0/1000 live-births (p<0.001)) and Northern regions (15.3 to 22.2/1000 live-births (p<0.001) but decreased in Western (23.7 to 17.0 (p<0.001)) and Eastern (15.0 to 8.9 (p<0.001) Uganda. Three districts had incidences of >50/1000 live-births.
Conclusion: Increase in incidence of EOS nationally points to a need to urgently address the quality of care for pregnant women and the quality of preventive measures for neonatal sepsis through timely maternal infection management across the country. Clean delivery and hospital environments should be emphasized. The heterogenous distribution of the incidence of neonatal-sepsis requires tailored root cause-analysis by health authorities in regions with consistently high neonatal-sepsis incidence. Prevention and treatment interventions in Central and Northern regions, as well as in the most affected districts should be strengthened.
Background
Neonatal sepsis caused 15% of neonatal deaths globally in 2018 and this impacts negatively on attainment of the Sustainable Development Goal to end preventable child deaths. There are approximately 1.3 million cases of neonatal sepsis annually, the bulk of which is in low-income countries especially those in Africa. Global data indicate a 3.5 times higher incidence in low-income countries and 1.8 times higher incidence in middle-income countries compared to high-income countries.
Although there is currently no concensus definition for neonatal sepsis, it is commonly referred to as a clinical syndrome that includes pneumonia and meningitis and characterised by bacterial infection in the first month of life (1, 2). Neonatal sepsis is categorized as either early-onset sepsis (EOS) if it occurs in the first seven days of life, or late-onset (LOS) sepsis if the infection occurs from 8 to 28 days of life. EOS is caused by intrapartum transmission of bacteria from the mother to the neonate (3-5), while LOS is usually acquired postnatally from the hospital or community environment (3). Risk factors for neonatal sepsis include: prematurity, low birth weight (below 2.5 kg), premature rupture of membranes, prolonged labour, caeserean section delivery, maternal infection, and lack of antenatal care (6-9). It is characterised by: temperature
instability, inability to breastfeed, seizures, respiratory distress, jaundice, vomiting, diarrhoea, abdominal distention, and diminished activity (10). Although the gold standard test for neonatal sepsis is a blood culture, in resource limited settings, cases are often diagnosed clinically.
In Uganda, neonatal sepsis is among the top three contributors to high neonatal mortality rate alongside other causes such as birth asphyxia, preterm birth complications, intrapartum related events, and other infections such as meningitis and pneumonia (11, 12). The recommended first-line treatment of neonatal sepsis in Uganda is administration of intravenous ampicillin and gentamycin and cephalosporins as second-line drugs (2, 13). Worryingly, despite new born health interventions, neonatal mortality rate (NMR) persisted at approximately 27/1,000 live births between 2002 and 2016, according to three successive demographic health survey reports (11) .
It is important that neonatal deaths decrease so that the 2030 national target of 12 or less new born deaths per 1,000 live births is met (14). There is paucity of epidemiological data on neonatal sepsis in Uganda. However, the Maternal Perinatal Death Surveillance and Response report for FY 2019/2020 attributed 12% of new born deaths in Uganda to neonatal sepsis (15) and a study done at Mulago National Referral hospital reported the case fatality rate from neonatal sepsis as 9.5% (16) . We describe the trends and spatial distribution of neonatal sepsis in Uganda between 2016 and 2020 so as to provide information that will guide interventions to reduce incidence of neonatal sepsis and sepsis related deaths.
Methods
Study setting
This study was done in Uganda, a country located in Sub-Saharan Africa. Uganda has a population of 34.6 million persons and a population growth rate of 3 percent according to the 2014 National Population and Housing Census (17). Uganda’s fertility rate is 5.4 children per woman, one of the highest in the world, and the crude birth rate is 38.7 per 1,000 population (11). There are 6, 937 health facilities in the country; Of these, 45.2 % are government owned, 14.4 % are private and Not for Profit (PNFP), 40.3 % are Private For Profit (PFP) and 0.1% (7) community-owned facilities. These health facilities are classified into seven levels; maternity services are provided at facilities from level 3 upwards (18). The recommended intravenous antibiotics for the management of neonatal sepsis are available at health facilities from level 3 and above (19).
Study design, neonatal sepsis surveillance, and data source
We performed a descriptive analysis of routinely reported surveillance aggregate data on in-patient neonatal sepsis using the District Health Information System version 2 (DHIS2) from 2016 to 2020. The DHIS2 is an electronic version of data from the Health Management Information System (HMIS). The HMIS is a paper-based reporting system where integrated health unit data on several conditions including neonatal sepsis are reported on a weekly and monthly basis. In the DHIS2, neonatal sepsis is categorised as in-patient and out-patient sepsis, which are further classified into early-onset (0-7 days) and late-onset (8-28 days) sepsis. Specifically, for the data extracted in this analysis, the flow of the data was from health units at level 3 to the health sub-district (level 4) and then to the district. At the district, data are entered by the district biostatistician by health facility level into DHIS2. Regional and national referral hospitals send data directly to the Ministry of Health. At the Ministry of health, data from all health facilities are collated and the national performance on each indicator is determined.
Study Population
The study population comprised of all records of new born babies aged 0-28 days (neonates) delivered at health facilities in Uganda between January 2016 and December 2020.
Study variables and data abstraction
For this analysis, we downloaded data elements on: Neonatal Sepsis at 0-7 days, Neonatal Sepsis at 8-28 days, and deliveries in a health unit (live births) from HMIS 108 which aggregates in-patient data. In-patient cases were considered because the computation of sepsis incidence would be more accurate since the denominator of live births is more readily available for health facility deliveries than home deliveries .We also downloaded data on the national reporting rate on neonatal sepsis for the years 2016 to 2020 from DHIS2 to determine whether fluctuations in reporting rates could have affected sepsis incidence. These data were then exported from DHIS2 to Microsoft Excel and then into Epi Info 7 for analysis.
Data Analysis
We calculated incidence rates of early-onset (0-7 days), late-onset (8-28 days), and total (0-28 days) neonatal sepsis from 2016 to 2020 at district, regional, and national levels. Incidence rates for total sepsis were also calculated at health facility level. Incidence rate was the number of sepsis cases divided by total live births per 1,000 live births. We demonstrated regional and national trends on line graphs and determined the significance of the change in trend using logistic regression in Epi-info version 7. We interpreted the odds ratios as the odds of increase or decrease in in-patient neonatal sepsis cases per 1,000 live births per year. Choropleth maps were drawn using QGIS version 3.6.3 to show the regional and district-level distribution of neonatal sepsis at district level.
Ethical considerations
This study utilized routinely reported surveillance data that did not have personal identifiers. We obtained permission to use the HMIS data from the Ministry of Health Resource Centre which is responsible for collating and storing health information. We stored the data in password-protected computers. The US Centers for Disease Control and Prevention (CDC) Center for Global Health determined this study was non-research with a main aim improving data use to guide health planning and practice.
Results
Trends of incidence of in-patient neonatal sepsis nationally, Uganda, 2016- 2020
Nationally, a total of 95,983 cases of in-patient total neonatal sepsis (early-onset plus late-onset sepsis) were reported from 2016 to 2020. Seventy five percent (71,262) of them were early-onset cases (0-7 days). On average, 1.7% of all live born neonates experienced sepsis (Table 1).
Table 1: Neonatal sepsis cases and total live births, Uganda, 2016-2020
| Year | LB† | Total sepsis | % among LB* | EOSπ | % among LB¶ | LOSβ | % among LB§ |
| 2016 | 959,078 | 16,717 | 1.7 | 11,249 | 1.2 | 5,468 | 0.6 |
| 2017 | 1,040,265 | 18,096 | 1.7 | 13,301 | 1.3 | 4,795 | 0.5 |
| 2018 | 1,123,279 | 19,328 | 1.7 | 14,838 | 1.3 | 4,490 | 0.4 |
| 2019 | 1,176,931 | 20,515 | 1.7 | 15,674 | 1.3 | 4,841 | 0.4 |
| 2020 | 1,205,995 | 21,327 | 1.8 | 16,200 | 1.3 | 5,127 | 0.4 |
†LB- Live births. *Percentage of total sepsis among live births. πEOS- Early-onset sepsis. ¶Percentage of EOS among live births. βLOS- Late-onset sepsis. §Percentage of LOS among live births
Overall incidence of total sepsis increased during 2016 to 2020 by an average of 0.004/1,000 live births per year (Figure 1), which was not statistically significant (P=0.133). This was on the backdrop of an almost constant reporting rate, which only increased in 2020 (Figure 1).
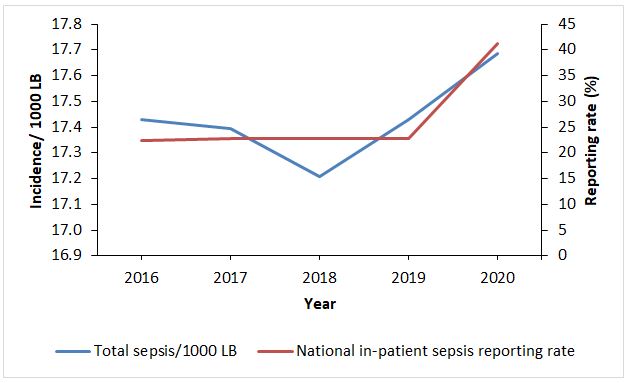
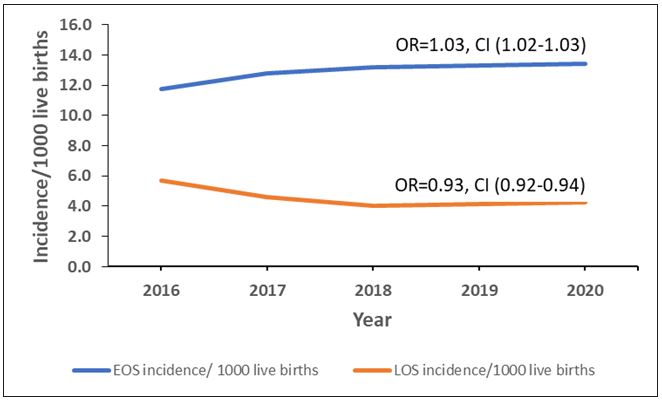
Early-onset sepsis increased from 11.7 to 13.4 cases/ 1,000 LB, with an average yearly increase of 3% (OR: 1.03, CI: 1.02- 1.03, p<0.0005) and late-onset sepsis declined from 5.7 to 4.3 cases/1000 LB with an average yearly decrease of 7% (OR: 0.93, CI: 0.92-0.94, p<0.0005) (Figure 2).
Trends of incidence of in-patient neonatal sepsis at regional level, Uganda, 2016- 2020
At regional level, early onset sepsis as well as total sepsis increased in Central and Northern regions but decreased in Western and Eastern Uganda. However, LOS only increased in Central region during the period while other regions registered decreases. These changes were statistically significant (Table 2).
Table 2: Significance of trends of neonatal sepsis incidence rates at regional level, Uganda, 2016-2020
| Variable | IR§ 2016 | IR 2020 | OR | 95% CI | P- value* |
| Total sepsis | |||||
| Central | 15.5 | 23 | 1.5 | 1.4- 1.6 | <0.001 |
| Northern | 15.3 | 22.2 | 1.2 | 1.2- 1.3 | <0.001 |
| Western | 23.7 | 17 | 0.7 | 0.7- 0.7 | <0.001 |
| Eastern | 15 | 8.9 | 0.6 | 0.6- 0.6 | <0.001 |
| Early-onset sepsis | |||||
| Central | 11.8 | 16 | 1.4 | 1.3- 1.4 | <0.001 |
| Northern | 8.3 | 16.8 | 2 | 1.9- 2.2 | <0.001 |
| Western | 15.4 | 14.1 | 0.9 | 0.9- 0.9 | <0.001 |
| Eastern | 10.9 | 7.3 | 0.7 | 0.6- 0.7 | <0.001 |
| Late-onset sepsis | |||||
| Central | 11.8 | 16 | 1.9 | 1.8-2.0 | <0.001 |
| Northern | 8.3 | 16.8 | 0.8 | 0.7-0.8 | <0.001 |
| Western | 15.4 | 14.1 | 0.3 | 0.3- 0.4 | <0.001 |
| Eastern | 10.9 | 7.3 | 0.4 | 0.4-0.5 | <0.001 |
*Significant association at p-value < 0.05
- IR= Incidence rate
Spatial distribution of neonatal sepsis incidence rates at district level, Uganda, 2016-2020
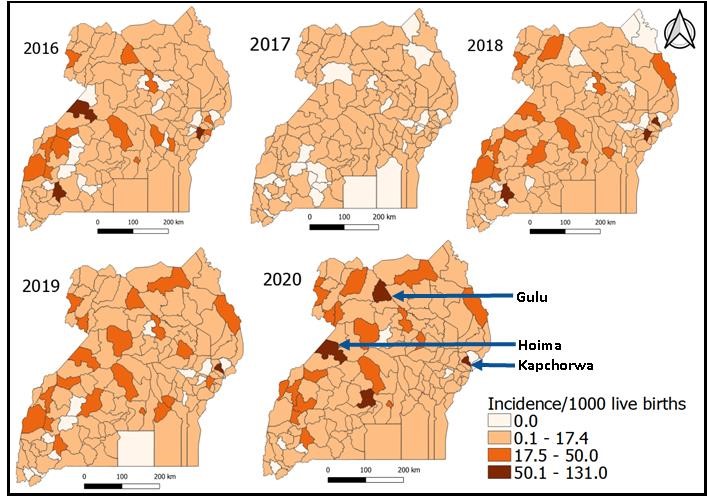
Despite Central and Northern regions having the highest early-onset sepsis across the years, the districts with the highest early-onset sepsis were not concentrated only in those regions. In 2017, districts generally showed much lower EOS incidence compared to rates in 2016 and other years. From 2018 to 2020, increasingly more districts had high EOS incidence in the range 17.5-50 cases/ 1,000 live births. The highest number of districts (5) with incidences of > 50 cases/1,000 live births occurred in 2020, and they included Gulu, Hoima and Kapchorwa (Figure 3).
Discussion
This study revealed that 1.7% neonates of all live births that occurred in Uganda between 2016 and 2020 developed sepsis, which is a preventable cause of neonatal morbidity and mortality. In an interrupted time series study conducted at an urban private hospital in Central Uganda, whereas the case fatality rate from intrapartum hypoxia and prematurity decreased significantly, that from neonatal sepsis increased from 1.9% in 2006 to 5.7 % in 2015 (20).
Another study done in Eastern Uganda showed that sepsis or pneumonia were the leading causes of all neonatal deaths accounting for 31% of such deaths and that these deaths are influenced by three delays. The delay by caretakers in identifying unwell neonates and seeking care contributed to 50% of neonatal deaths, delay in receiving quality care at the health facility contributed 30%, while transport delay to the health facility contributed 20% (21).
Therefore, strategies that address the three delays could result in timely intervention for neonates with sepsis so as to prevent adverse outcomes such as death. Nearly three quarters of sepsis cases in this study were early-onset, that is, they occurred within the first 7 days of newborn life. This finding is similar to the 2.6 fold higher incidence of EOS than LOS that was reported globally and to the 80% EOS incidence of all sepsis that was found in South Western Uganda (22, 23).
Sankar, Natarajan (24) showed that one-half of sepsis-related deaths in developing countries occur in the first week of life, whereas another study in Eastern Uganda revealed that 78% of neonatal deaths occurred in the first week (21). The early neonatal period represents a time when neonates born at health facilities are exposed to the health facility environment. Neonates are susceptible to sepsis from health care associated infections which occur more commonly in resource limited settings; health-care associated infections account for 1 in 4 sepsis cases in hospitals (25).
Early onset sepsis reflects issues of quality of care which include infrastructure limitations that hinder adequate care of pregnant women and neonates, suboptimal use of preventive measures such as early diagnosis of maternal infection for prompt treatment before it spreads to the neonate, and poor management of maternal and neonatal infections and their complications (26). Since EOS usually results from infection acquired in utero or during the birth process, the period around birth represents an important time at which to intervene to control neonatal sepsis.
In this study, we observed mixed trends by region in neonatal sepsis with the Central and Northern regions showing increasing trends while declining trends were observed in Eastern and Western Uganda. The high incidence in Central region may possibly be due to the high population density and the region also has the highest concentration of health facilities (18). While total sepsis incidence appeared to increase, the reporting rate for neonatal sepsis remained almost constant from 2016 to 2019. However, in 2020, the reporting rate increased.
The increase in total sepsis in 2020 might be due to the increased reporting rate in the same year. Increased sepsis in 2020 is also probably due to emergence of COVID-19 which increased home births hence sepsis. COVID-19 also caused delays in mothers reaching hospital hence prolonged labour which is a risk factor for infection. One of the attributable factors for the reduction in Eastern Uganda could be the prompt adherence by care takers of newborns to community health workers’ facility referral advice. (27). Early identification of danger signs of infection presents an opportunity for timely interventions thus decreasing adverse outcomes.
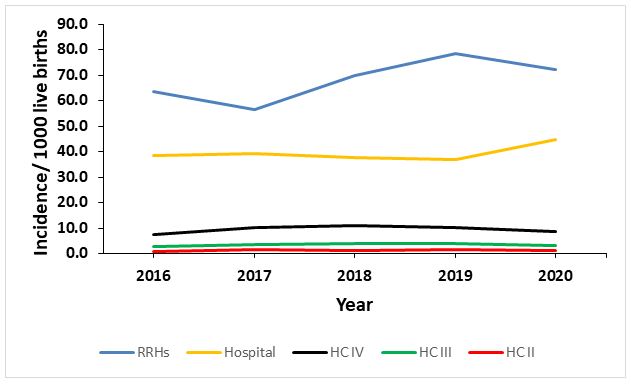
Health facility level analysis showed that regional referral hospitals had the highest incidence of neonatal sepsis which is expected given that lower level health facilities refer complicated cases of neonatal infections to them. Hospitals also have a higher availability of drugs for treating neonatal sepsis than lower level facilities (28). Four of the five high burden districts (Mbale, Gulu, Hoima, and Mbarara) each have a regional referral hospital. Presence of a referral hospital in these districts implies that they receive cases from neighbouring districts in their respective regions which could be an additional factor for high incidence rates observed in these districts.
The financial burden of neonatal sepsis in sub-Saharan Africa has been described (29). Investing in prevention and effective case management of neonatal sepsis has important benefits including the prevention of nervous system complications that result in serious permanent disability (30). There is need for health workers to scale up infection prevention and controls practices in the most affected settings including in Central and Northern Uganda, the most affected districts, and at regional referral hospitals. Guidelines to health workers to improve essential maternal and newborn care were launched in October 2021 by the Ministry of Health.
Widespread roll out and implementation of these guidelines by the Ministry of Health to health facilities will likely improve maternal and neonatal outcomes. Routine care for all pregnant women and neonates includes ensuring a clean delivery environment (31). Health workers should continuously strive to create a conducive environment for child birth by ensuring a clean delivery environment. Antenatal care visits should be harnessed as points of education of pregnant women on neonatal sepsis including its causes, danger signs, and prevention strategies. Mothers should be encouraged to identify signs of infection they may be harbouring especially towards term pregnancy so that they seek treatment early.
Study Limitations
The data in the district health surveillance system are aggregated data and can only be used to analyze data at aggregate level. Health care access bias where the numbers we see are only those who make it to the health facilities could have led to underestimation of the burden of neonatal sepsis. In addition, this analysis includes only in-patient DHIS2 sepsis data and excludes out-patient department data which would likely include a greater proportion of neonatal sepsis from home births. These factors could have led to underestimation of the burden of neonatal sepsis. Also, reporting rates for the study period were low so there is a possibility that neonatal sepsis cases were under represented. Moreover, for each of the years, some districts did not report neonatal sepsis cases (not even zero reporting). For such districts, incidence rate of neonatal sepsis per 1,000 live deliveries could not be computed. We studied in-patient cases and so have missed out on the true burden of late-onset cases in the community.
Acknowledgements
The authors would like to thank the Ministry of Health Division of Health information for permitting us to use these data, and the Reproductive Health Department of the Ministry of Health as well as the Uganda Public Health Fellowship Program for the technical support.
References
- Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. Bmj. 2007;335(7625):879-83.
- Ministry of Health. Essential Maternal and Newborn Clinical Care Guidelines for Uganda. In: Department RaCH, editor. Kampala, Uganda: Ministry of Health; 2021.
- Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatric Clinics. 2013;60(2):367-89.
- Cortese F, Scicchitano P, Gesualdo M, Filaninno A, De Giorgi E, Schettini F, et al. Early and late infections in newborns: where do we stand? A review. Pediatrics & Neonatology. 2016;57(4):265-73.
- Mukhopadhyay S, Puopolo KM, editors. Risk assessment in neonatal early onset sepsis. Seminars in perinatology; 2012: Elsevier.
- Beletew B, Kassie A, Getu MA. Neonatal sepsis and its associated factors in East Africa: a systematic review and meta-analysis, 2019. 2019.
- Chan GJ, Lee AC, Baqui AH, Tan J, Black RE. Risk of early-onset neonatal infection with maternal infection or colonization: a global systematic review and meta-analysis. PLoS Med. 2013;10(8):e1001502.
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1789-858.
- Kayom VO, Mugalu J, Kakuru A, Kiguli S, Karamagi C. Burden and factors associated with clinical neonatal sepsis in urban Uganda: a community cohort study. BMC pediatrics. 2018;18(1):1-8.
- Tesina BL. Neonatal Sepsis 2020 [Available from: https://www.msdmanuals.com/professional/pediatrics/infections-in-neonates/neonatal-sepsis.
- UBOS, ICF. Uganda Demographic and Health Survey 2016: Key Indicators Report. Kampala, Uganda:UBOS Rockville, Maryland, USA:UBOS and ICF: Uganda Bureau of Statistics (UBOS) ICF; 2017.
- UNICEF. Levels and Trends in Child Mortality. New York, USA; 2019.
- Ministry of Health. Uganda Clinical Guidelines 2016 National Guidelines for Management of Common Conditions. In: Department P, editor. Kampala, Uganda: Ministry of Health; 2016.
- Lawn J, Kinney M, Blencowe H. Every newborn. An executive summary for The Lancet’s Series. Lancet. 2014;384(9938):1-8.
- Ministry of Health. The National Annual Maternal and Perinatal Death Surveillance and Response (MPDSR) Report FY 2019/2020. Kampala, Uganda; 2020.
- Tumuhamye J, Sommerfelt H, Bwanga F, Ndeezi G, Mukunya D, Napyo A, et al. Neonatal sepsis at Mulago national referral hospital in Uganda: Etiology, antimicrobial resistance, associated factors and case fatality risk. PLoS One. 2020;15(8):e0237085.
- UBOS. National Population and Housing Census 2014. Kampala, Uganda: Uganda Bureau of Statistics; 2016.
- Ministry of Health. National Health Facility Master List 2018. Kampala, Uganda: Ministry of Health; 2018.
- Ministry of Health. Essential Medicines and Health Supplies List for Uganda (EMHSLU) 2016. Kampala, Uganda: Ministry of Health; 2016.
- Kirabira VN, Aminu M, Dewez JE, Byaruhanga R, Okong P, van den Broek N. Prospective study to explore changes in quality of care and perinatal outcomes after implementation of perinatal death audit in Uganda. BMJ open. 2020;10(7):e027504.
- Waiswa P, Kallander K, Peterson S, Tomson G, Pariyo GW. Using the three delays model to understand why newborn babies die in eastern Uganda. Tropical medicine & international health. 2010;15(8):964-72.
- Fleischmann C, Reichert F, Cassini A, Horner R, Harder T, Markwart R, et al. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Archives of Disease in Childhood. 2021.
- Kiwanuka J, Bazira J, Mwanga J, Tumusiime D, Nyesigire E, Lwanga N, et al. The microbial spectrum of neonatal sepsis in Uganda: recovery of culturable bacteria in mother-infant pairs. PLoS One. 2013;8(8):e72775.
- Sankar MJ, Natarajan C, Das R, Agarwal R, Chandrasekaran A, Paul V. When do newborns die? A systematic review of timing of overall and cause-specific neonatal deaths in developing countries. Journal of perinatology. 2016;36(1):S1-S11.
- World Health Organization. Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. Geneva; 2020.
- Chou D, Daelmans B, Jolivet RR, Kinney M, Say L. Ending preventable maternal and newborn mortality and stillbirths. Bmj. 2015;351.
- Nalwadda CK, Waiswa P, Kiguli J, Namazzi G, Namutamba S, Tomson G, et al. High compliance with newborn community-to-facility referral in eastern Uganda:. an opportunity to improve newborn survival. PLoS One. 2013;8(11):e81610.
- Nalwadda CK, Peterson S, Tomson G, Guwatudde D, Kiguli J, Namazzi G, et al. Health system preparedness for newborn care: a health facility assessment in rural Uganda. BMC health services research. 2014;14(2):1-.
- Ranjeva SL, Warf BC, Schiff SJ. Economic burden of neonatal sepsis in sub-Saharan Africa. BMJ global health. 2018;3(1):e000347.
- Warf BC, Dagi AR, Kaaya BN, Schiff SJ. Five-year survival and outcome of treatment for postinfectious hydrocephalus in Ugandan infants. Journal of Neurosurgery: Pediatrics. 2011;8(5):502-8.
- Gabrysch S, Civitelli G, Edmond KM, Mathai M, Ali M, Bhutta ZA, et al. New signal functions to measure the ability of health facilities to provide routine and emergency newborn care. PLoS medicine. 2012;9(11):e1001340.


Comments are closed.