Epidemiological characteristics and trends of measles cases reported through the case-based surveillance system, Uganda, 2016 – 2020
Authors: Zainah Kabami1*, Brenda Nakafeero Simbwa1, Saudah Namubiru Kizito1, Brian Agaba1, Joshua Kayiwa2, Daniel Kadobera1, Lilian Bulage1, Richard Migisha1 Institutional affiliations: 1Uganda Public Health Fellowship Program-Uganda National Institute of Public Health, Kampala, Uganda; 2National Public Health Operations Center, Kampala, Uganda Correspondence*: Tel: +256758630580, Email: zkabami@musph.ac.ug; zkabami@uniph.go.ug
Summary
Background: Despite 95% measles vaccine coverage in Uganda, measles is the most commonly-reported outbreak in the country. In 2015, as part of the regional measles elimination goal, World Health Organization (WHO) targeted ≥95% vaccination coverage and a measles incidence of <1 confirmed case per million population per year. We describe the epidemiological characteristics and trends of measles cases reported through the case-based surveillance system in Uganda to track progress towards elimination.
Methods: We used data from the active case-based measles surveillance system at the Uganda Virus Research Institute for 2016–2020. A suspected case was any person with fever and maculopapular (non-vesicular) generalized rash and cough, coryza or conjunctivitis diagnosed by a clinician OR any person in whom a clinician suspected measles. Laboratory-confirmed cases were suspected cases with IgM-positive results for measles. Epidemiologically-linked cases were suspected cases without laboratory confirmation but linked in place, person, and time to a confirmed case. Clinically compatible cases were suspected cases without adequate investigation. We calculated measles incidence during the study period and disaggregated it by year, age-group, sex, region, and vaccination status.
Results: Among 5,047 cases, 2,120 (42%) were clinically compatible, 1,715 (34%) were laboratory-confirmed, and 1,212 (24%) were epidemiologically linked; six (0.1%) died. Half (n=2,595, 51%) were male. Children <5 years were more affected than persons aged ≥5 years (451 vs 65/1,000,000) (p<0.0001). In total, 3,367 (67%) were vaccine-eligible; of these, 1,821 (54%) were unvaccinated. Annual incidence was 7, 29, 66, 23, and 27/1,000,000 from 2016–2020; incidence reduced after a national vaccination campaign in 2019. Overall incidence was higher among the unvaccinated than vaccinated (40 vs 36/1,000,000) (p<0.0001).
Conclusion: The measles incidence across the study period was consistently >1/1000,000, putting Uganda off track for measles elimination. Only half of the cases were vaccinated, which might suggest an overestimation of vaccination history or a challenge with vaccine effectiveness. There is likely need for intensification of regular mass measles vaccination and further studies to investigate vaccine effectiveness.
Introduction
Measles remains a significant cause of morbidity and mortality among young children globally despite the availability of a safe and effective vaccine [1]. While vaccination has considerably reduced deaths attributable to measles worldwide, it is still common in many low-and-middle-income countries, particularly in parts of Africa and Asia [2]. Globally, an estimated 9.7 million cases and over 140,000 deaths were reported in 2018, almost 50% of which were attributable to only four countries in sub-Saharan Africa [2].
In Uganda, measles is the most commonly reported outbreak despite 95% national vaccine coverage [3]. Measles surveillance in Uganda is conducted through the Integrated Disease Surveillance and Response (IDSR) framework . Within this framework, measles is listed as one of the immediately notifiable diseases that all health facilities in the country are required to report to the next level using the standard case definition specified in the IDSR guidelines. In the passive surveillance system, data is collected from suspected measles cases during visits to health facilities, and routinely reported as part of a general aggregate summary along with other notifiable diseases [5].
This is then submitted on a weekly, monthly, and quarterly basis to the Ministry of Health (MoH) through the electronic Health Management Information System (eHMIS) [4]. To complement the passive surveillance system, Uganda adopted a case-based surveillance system in 2003 where a suspected measles case is to be investigated with laboratory testing for measles-specific immunoglobulin (IgM) at the national EPI lab of the Uganda Virus Research Institute (UVRI) [6]. The UVRI receives blood specimen from all health facilities through the laboratory sample transport hub system and it must be accompanied with a case-based form or line list filled in by the health worker investigating [7]. This information is then entered into excel and shared with key stakeholders for operational use.
In 2015, as part of the regional measles elimination goal, World Health Organization (WHO) AFRO region targeted ≥95% national vaccination coverage, and a measles incidence of <1 confirmed case per million population per year. In view of these targets, we describe the epidemiological characteristics, and trends of measles cases reported through the case-based surveillance system in Uganda to track progress towards elimination.
Methods
Study setting, design, and data source
We conducted a descriptive analysis using measles case-based surveillance data, 2016-2020, generated by all health facilities from Uganda at the Uganda Virus Research Institute (UVRI). Uganda had an estimated population of 46,210,758 in 2022 and a growth rate of 3.1% per annum [8]. The life expectancy on average is 64 years with an infant mortality rate of 43 per 1,000. Administratively, Uganda is stratified into 135 districts; with a health system comprised of decentralized healthcare services, overseen by district health teams across all districts and the central MoH [9]. The data reported through the case-based measles surveillance system comprises demographic information, clinical history, specimen submitted, investigator details, and results. The first four sections are completed by the investigator while the results section with final classification is completed upon testing by the laboratory staff.
As per the Uganda National Technical Guidelines for IDSR [10]; a suspected measles case is any person with fever and maculopapular (non-vesicular) generalized rash and cough, coryza or conjunctivitis (red eyes) as diagnosed by a clinician OR any person in whom a clinician suspected measles. A laboratory confirmed measles case is defined as suspected measles cases which had laboratory results indicating measles IgM positive. Cases confirmed by epidemiological link are suspected measles cases that did not have a blood specimen taken for serologic confirmation and were linked in place, person and time to a laboratory confirmed case. Clinically compatible cases are suspected measles cases which had not been adequately investigated. Discarded measles cases are suspected cases with negative laboratory results for measles IgM. We considered the sum of laboratory-confirmed, epidemiologically linked, and clinically compatible as measles confirmed cases, as recommended by WHO.
Study variables and data analysis
Data on measles cases for each of the years (2016-2020) was merged into one excel sheet for cleaning and analysis. Descriptive statistics including frequency, proportions, and percentages were calculated and tabulated.
We used a line graph to illustrate trends of cases, disaggregated by classification for each of the study years. We drew choropleth maps using QGIS to show the annual incidence of measles in the various districts. We divided the annual sum of measles cases by the corresponding year’s projected population estimates of reporting districts based on the 2014 census, and calculated the annual incidence rate per million.
We calculated measles incidence and disaggregated it by year, region, vaccination status, and age-group. We calculated age-specific measles incidence rates using reported cases within that age-group as the numerator and the respective projected population estimates as the denominator. The incidence rates were presented on a trend line graph.
We also plotted cases using five-year age-groups against vaccination status and corresponding incidence for each of the study years.
Ethical approval
Our study utilized routinely generated surveillance data submitted to the UVRI. The Uganda Public Health Fellowship Program is part of the National Rapid Response Team, and has been granted permission to access and analyse surveillance data to inform decision making in the control and prevention of outbreaks and public health programming.
Additionally, the MOH has also granted the program permission to disseminate the information through scientific publications. We sought administrative permission to access the data from the Uganda National Expanded Program on Immunization (UNEPI) and UVRI. We stored the abstracted data set in a password-protected computer.
In addition, the Office of the Associate Director for Science, U.S. Centers for Disease Control and Prevention, determined that this study was not a human subjects research with the primary intent of improving use of surveillance data to guide public health planning and practice.
Results
Distribution of measles cases by final laboratory categorization as reported from the case-based surveillance system, Uganda, 2016-2020
During the five-year study period (January 2016 – December 2020), a total of 5,047 cases were identified; 2,120 (42%) were clinically compatible, 1,715 (34%) were laboratory-confirmed, and 1,212 (24%) were epidemiologically linked (Table 1).
Table 1: Distribution of measles cases by final laboratory categorization as reported from the case-based surveillance system, Uganda, 2016-2020
| Year | Number of districts reporting measles cases | Laboratory confirmed (%) | Epidemiological link (%) | Clinically compatible (%) | Total |
| 2016 | 40 | 129 (51) | 10 (4) | 113 (45) | 252 |
| 2017 | 68 | 180 (18) | 482 (48) | 336 (34) | 998 |
| 2018 | 119 | 640 (25) | 351 (14) | 1,589 (61) | 2,580 |
| 2019 | 100 | 626 (70) | 203 (22) | 78 (8) | 907 |
| 2020 | 43 | 140 (45) | 166 (54) | 4 (1) | 310 |
| Total | 1,715 | 1,212 | 2,120 | 5,047 | |
Characteristics of measles cases reported from the case-based surveillance system, Uganda, 2016 – 2020
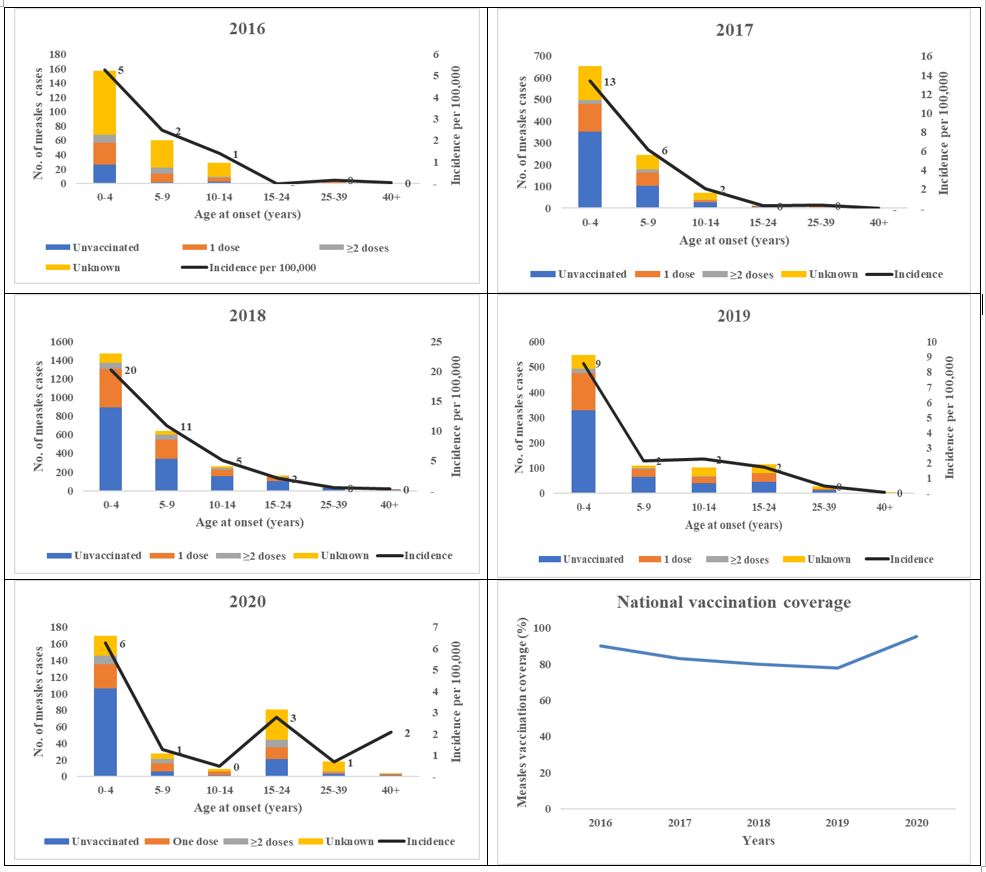
Of the 5,047, fifty-one percent of the cases were male. Median age was 5 years (Range: 0-67). Children in the 0-4 age-group formed the majority of cases (59%) followed by the 5-9 age-group (22%). The majority were from rural districts (91%). Seventy-six percent were outpatient visits, while 24% were inpatients among whom 6 died (Case Fatality Rate: 0.1%). In total, 3,367 (67%) were vaccine-eligible; of these, 1,821 (54%) were unvaccinated (Table 2).
Table 2: Characteristics of measles cases reported from the case-based surveillance system, Uganda, 2016 – 2020
| Characteristic | Frequency (N=5,047) | Percentage (%) |
| Sex | ||
| Male
Female Age-group 0-4 5-9 10-14 15-24 25-39 40≥ Geographic area Rural Urban Department of care Outpatient Inpatient Missing Disease outcome Alive Dead Vaccination status (eligible) Unvaccinated ≥ 1 dose |
2,595
2,452
2,987 1,088 475 378 97 22
4,617 430
3,849 1,196 2
5,041 6
1,821 1,546 |
51
49
59 22 10 7 2 0
91 9
76 24 0
100 0
53 47 |
Dates of onset ranged from January 2016 to November 2020; In 2017, the incidence gradually increased and peaked towards the end of the first quarter of 2018 (Figure 1).
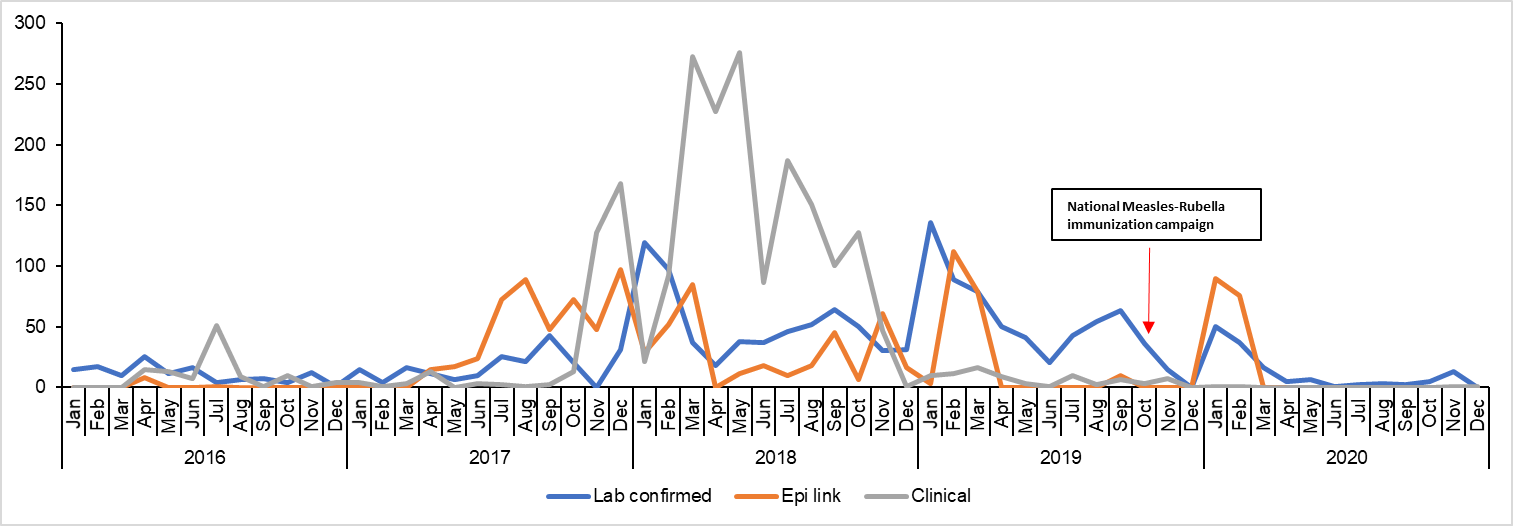
Annual incident rates of measles cases by age-group, case-based surveillance system, Uganda, 2016-2020
The overall incidence for the study period was 133/1,000,000. Annual incidence increased from 7/1,000,000 in 2016 to 29/1,000,000 in 2017, and was highest in 2018 at
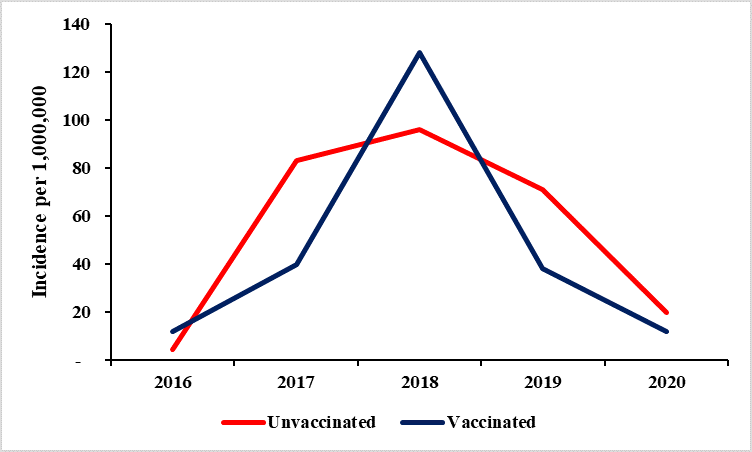
66/1,000,000. Incidence reduced to 23/1,000,000 following a national vaccination campaign in 2019, and slightly increased to 27/1,000,000 in 2020. Children <5 years were more affected than persons aged ≥5 years (Incidence: 451 vs 65/1,000,000) (p<0.0001), and consistently reported a much higher incidence rate compared to the overall, and those aged ≥5 years (Figure 2).
Age distribution, vaccination status, and incidence of measles cases from the case-based surveillance system, Uganda, 2016-2020
Age and incidence generally had an inverse relationship, as age increased, the incidence rate went down and vice versa. However, in 2020, there was a higher incidence rate in the 15-24 age-group in comparison to the 5-14 age group (Figure 4).
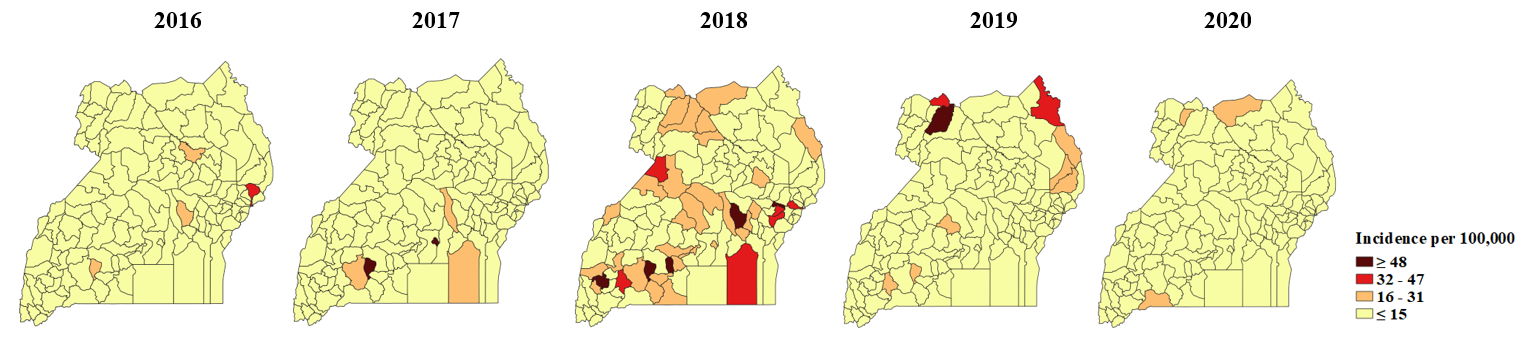
Incidence of measles cases among persons aged less than five years by district, case-based surveillance system, Uganda, 2016-2020
No particular region registered a consistently high incidence rate over the years. However, the Central region featured in the years 2016-2019, and Lyantonde District consistently reported an incidence rate of ≥16 per 100,000 from 2016-2019. There was a countrywide outbreak in 2018 that affected all regions but the central and eastern regions had more districts reporting higher incidence rates that other regions (Figure 5).
Discussion
This study set out to describe the epidemiological characteristics and trends of measles cases reported through the national case-based surveillance system in Uganda (2016-2020) to track progress towards elimination.
With a 1:1 ratio, there was no discernable difference in sex among the cases, meaning it was not necessarily a risk factor for measles; a finding consistent with other studies describing the epidemiology of measles in the African region [11, 12]. On the other hand, we found a significant difference in geographic location of cases. An overwhelming majority of them came from rural settings, as has been reported in previous outbreaks in Kasese, Lyantonde, Nakaseke, and Buvuma districts of Uganda [4, 13-15], and in other countries both within and outside the African region [5, 16]. This reinforces the general understanding that measles, like most infectious diseases, is largely correlated with a low standard of living.
The annual incidence ranged from 7-66 per million population across the study years. The African Region of the WHO developed a strategic plan, which included a number of targets to be achieved by 2020, as part of the measles elimination goal [17]. This included achieving a measles incidence of less than one confirmed measles case reported per million population per year [17]. Based on our results, Uganda was off track for elimination as our incidence rate was consistently far higher than the recommended target.
Children aged <5 years consistently reported a higher incidence rate than those aged ≥5-years emphasizing the vulnerability of young children to measles. Measles tends to disproportionately affect children under five years of age, who are who are often the most susceptible to severe complications and death [18]. These findings are consistent with results of studies conducted on the epidemiology of measles in Uganda [13, 19], and globally [20, 21]. This age-related discrepancy reinforces the importance of prioritizing measles vaccination for young children.
Of the total cases, 67% were eligible for vaccination, with 54% of these individuals remaining unvaccinated. This denotes an immunity gap, possibly due to the
accumulation of susceptible individuals, and may explain why Measles outbreaks are the most commonly reported in Uganda. This finding highlights the need for enhanced efforts to reach eligible but unvaccinated individuals, and improve vaccination coverage to reduce measles incidence [5]. Notably, there were variations in incidence among the vaccinated and unvaccinated populations in different years, with higher incidence among the vaccinated in 2016 and 2018, suggesting potential gaps in vaccine effectiveness or coverage.
Study limitations
Our study had some limitations. Given that our analysis was restricted to case-based data, it represents a lower estimate of the total number of measles cases, particularly clinically compatible cases, in comparison to the aggregate summaries reported through the electronic Health Management Information System as seen in similar studies in Nigeria and Ethiopia [22, 23]. However, the laboratory confirmed cases depict an accurate national picture of confirmed cases as measles samples from all parts of the country are submitted to UVRI for analysis.
Secondly, for those whose data on vaccination status was available, it relied on recall in absence of a vaccination card and could have led to over or under-estimation of vaccination status.
Conclusion
The measles incidence across the study period was consistently >1/1000,000, putting Uganda off track for measles elimination. Only half of the cases were vaccinated, which might suggest an overestimation of vaccination history or a challenge with vaccine effectiveness. There is likely need for intensification of regular mass measles vaccination and further studies to investigate vaccine effectiveness and/or coverage.
Conflict of interest
The authors declare that they had no conflict of interest.
Author contribution
Zainah Kabami led the conception, design, analysis, interpretation of the study and wrote the draft bulletin; Zainah Kabami, Brenda Nakafeero Simbwa, Saudah Namubiru Kizito, Brian Agaba, and Joshua Kayiwa supported data analysis; Richard Migisha and Daniel Kadobera reviewed the report and Lilian Bulage reviewed the report and bulletin and provided technical support in improving the write up.
Acknowledgements
The authors thank the staff of the Public Health Fellowship Program for the technical support and guidance offered during this study. The authors also extend their appreciation to the National Public Health Emergency Operations Centre (NPHEOC) and the UNEPI for their permission and support in assessing the data utilized in this study.
Copyright and licensing
All materials in the Uganda Public Health Bulletin are in the public domain and may be used and reprinted without permission; citation as to source; however, is appreciated.
Any article can be reprinted or published. If cited as preprint, it should be referenced in the original form.
References
- Bester, J.C., Measles and measles vaccination: a review. JAMA pediatrics, 2016. 170(12): p. 1209-1215.
- WHO. Measles. 2023 March 20, 2023 [cited 2022 May 15].
- UNICEF. Over 20 million doses of Measles and Rubella vaccine arrive in Uganda. 2022 July 2019 [cited 2022 May 20].
- Nsubuga, F., et al., Positive predictive value and effectiveness of measles case-based surveillance in Uganda, 2012-2015. Plos one, 2017. 12(9): p. e0184549.
- Bolongaita, S., et al., Modeling the relative risk of incidence and mortality of select vaccine-preventable diseases by wealth group and geographic region in Ethiopia. PLOS Global Public Health, 2022. 2(8): p. e0000819.
- Mbabazi, W.B., et al., Achieving measles control: lessons from the 2002–06 measles control strategy for Uganda. Health policy and planning, 2009. 24(4): p. 261-269.
- Mensah, E.A. and S.O. Gyasi, Measles-Rubella Positivity Rate and Associated Factors in Pre-Mass and Post-Mass Vaccination Periods: Analysis of Uganda Routine Surveillance Laboratory Data. Advances in Public Health, 2022. 2022.
- UBOS. Mid Year Population Projections, National and Sub National. 2022 [cited 2022 July 2022].
- MOLG. Supporting Institutional Excellence and Wealth Creation. 2016 [cited 2022 July 20].
- Uganda, M.o.H. National Technical Guidelines for Integrated Disease Surveillance and Response, third edition. 2021 2021 [cited 2022 January 23 2023].
- Gutu, M.A., et al., Epidemiology of measles in Oromia region, Ethiopia, 2007-2016. The Pan African Medical Journal, 2020. 37.
- Ntshoe, G.M., et al., Measles outbreak in South Africa: epidemiology of laboratory-confirmed measles cases and assessment of intervention, 2009–2011. PLoS One, 2013. 8(2): p. e55682.
- Biribawa, C., et al., Measles outbreak amplified in a pediatric ward: Lyantonde District, Uganda, August 2017. BMC infectious diseases, 2020. 20(1): p. 1-8.
- Nguna, J., Estimating the Costs of Responding to a Measles Outbreak: Buvuma Islands, Lake Victoria, Uganda, February-May 2017. J Interval Epidemiol Public Health, 2022. 5(1): p. 1.
- Walekhwa, A.W., et al., Measles outbreak in Western Uganda: a case-control study. BMC Infectious Diseases, 2021. 21(1): p. 1-9.
- Douangboupha, V., et al., Factors contributing to a measles outbreak in a hard-to-reach rural village in Xaisomboun Province, Lao People’s Democratic Republic: Measles outbreak in Xaisomboun, Lao PDR. Western Pacific Surveillance and Response, 2022. 13(3): p. 8-8.
- WHO, African regional guidelines for measles and rubella surveillance. 2015, World Health Organization Regional Office for Africa.
- Donadel, M., et al., Risk factors for measles deaths among children during a Nationwide measles outbreak–Romania, 2016–2018. BMC Infectious Diseases, 2021. 21(1): p. 1-10.
- Nsubuga, E.J., et al., Measles outbreak in Semuto Subcounty, Nakaseke District, Uganda, June–August 2021. IJID Regions, 2022. 5: p. 44-50.
- Baptiste, A.E.J., et al., Trends in measles incidence and measles vaccination coverage in Nigeria, 2008–2018. Vaccine, 2021. 39: p. C89-C95.
- Yousif, M., et al., Measles incidence in South Africa: a six-year review, 2015—2020. BMC public health, 2022. 22(1): p. 1647.
- Fatiregun, A.A., A.S. Adebowale, and A.F. Fagbamigbe, Epidemiology of measles in Southwest Nigeria: an analysis of measles case-based surveillance data from 2007 to 2012. Transactions of the Royal Society of Tropical Medicine and Hygiene, 2014. 108(3): p. 133-140.
- Hassen, M.N., et al., Epidemiology of measles in the metropolitan setting, Addis Ababa, Ethiopia, 2005–2014: a retrospective descriptive surveillance data analysis. BMC Infectious Diseases, 2018. 18(1): p. 1-8.


Comments are closed.