Effect of ambient air pollution during pregnancy on preterm births: time-to-event analysis in Kampala City, Uganda, October 2021– September 2022
Authors: Mackline Ninsiima1*, Alex Ndyabakira2, Asiphas Owaraganise3, Sarah Zalwango2, Dorothy Aanyu1, Thomas Kiggundu1, Peter Waiswa4, Lynn Atuyambe5, Daniel Kadobera1, Lilian Bulage1, Richard Migisha1, Alex Riolexus Ario1, Daniel Ayen Okello2 Institution affiliations: 1Uganda Public Health Fellowship Program, Uganda National Institute of Public Health, Kampala, Uganda, 2Kampala Capital City Authority, Kampala, Uganda, 3Department of Obstetrics and Gynecology, Mbarara University of Science and Technology, Mbarara, Uganda,4Department of Community Health and Behavioral Sciences, School of Public Health, Makerere University, Uganda, 5Department of Health Policy, Planning and Management, School of Public Health, Makerere University, Uganda, Correspondence*: Tel: +256 787 819 496, Email: nmackline@musph.ac.ug
Summary
Introduction: Gestational exposure to fine particulate matter (PM2.5) has been associated with adverse birth outcomes. We investigated the effect of gestational PM2.5 exposure on preterm birth (PTB) in Kampala City, October 2021–September 2022.
Methods: We conducted a retrospective cohort study among mothers with singleton pregnancies ≥28 weeks of gestation who resided in Kampala City throughout their pregnancy, and delivered at Kawempe National Referral Hospital. PTB was defined as delivery before 37 weeks from first day of the last menstruation period. We estimated gestational PM2.5 exposure based on average
PM2.5 concentration obtained from the nearest Clarity© Node Solar–Powered monitor to the primary residence during pregnancy. We applied Mann Whitney U test to compare gestational PM2.5 exposure between pregnant mothers who had PTB and term birth. Gestational exposure to PM2.5 concentration was considered as the principal predictor of PTB, and we subsequently adjusted for potential covariates using multivariate regression with the Cox proportional hazards model. We assessed statistical significance of the multiplicative interaction by antepartum complications using Wald’s Chi- squared test.
Results: Among 1,540 births, 229 (15%) were preterm. Overall, average gestational PM2.5 exposure was 66μg/m3 (range: 45–75μg/m3). Significant difference in gestational PM2.5 exposure was observed between pregnant mothers who had preterm birth and those who delivered at term (p=0.002). For every unit increase in average gestational PM2.5 exposure, risk of incidence of preterm birth increased by 3% (HR=1.03, 95%CI: 1.01–1.05). Pregnant mothers who developed hypertensive disorders had 61% (HR=1.61, 95%CI: 1.05–2.48) higher risk of experiencing PTB compared to their counterparts.
There was no statistically significant difference in stratum specific hazard ratios between gestational PM2.5 exposure and incidence of preterm birth by antepartum complications.
Conclusion: We observed significant impact of PM2.5 concentration on incidence of PTB in Kampala City. Efforts aimed at reducing preterm births should also prioritize mitigation of air pollution to improve maternal and child health.
Background
Fine particulate matter (PM2.5) is among the health-damaging air pollutants that pose adverse risks to humans due to its small size and diameter: which easily permit penetration into invasive systems [1]. PM2.5 has been recommended as the best measure of air quality due to its prevalence in the environment and broad range of health effects with levels >15 µg/m3 being associated with adverse consequences. Gestational exposure to PM2.5 increases the risks for preterm birth, defined as delivery before 37 weeks or 259 days from the first day of a pregnant woman’s last menstruation to delivery.
Preterm birth is categorized into extremely preterm birth (<28 weeks), very preterm birth (28 to <32 weeks), and moderate preterm birth (32 to <37 weeks). Preterm births have been substantially linked to an increase in neonatal and infant mortality and development of chronic physical and neurological morbidity among the survivors compared to term births. The prevalence of preterm birth attributed to gestational PM2.5 exposure ranges from 12% to 24% worldwide [2]. A study conducted in Africa found a significant association between gestational exposure to PM2.5 and the incidence of preterm birth with an odds ratio of 1.08 (95% CI: 1.01, 1.16) [3]. Despite the fact that the direct causative mechanism is unknown, it is hypothesized that PM2.5 affects transplacental oxygen and nutrient transport thus placental inflammation, oxidative stress, and blood coagulation which may limit intrauterine fetal growth.
Cities are more prone to poor air quality compared to non-urban areas. This is attributed to high population density, exhaust emissions from vehicles and industries, infrastructure construction, open fuel and solid waste burning. As of 2021, Kampala City was among the cities with the highest levels of PM2.5, exceeding the annual WHO recommended air quality PM2.5 levels by 5 to 7 times. [4]. However, limited evidence has been presented about the association between PM2.5 exposure and preterm births in this city. Understanding the impact of air quality on the incidence of preterm births would be a great initiative towards influencing the implementation of evidence-based air quality control strategies that address adverse birth outcomes. We investigated the impact of PM2.5 exposure during pregnancy on preterm birth (PTB) in Kampala City, Uganda, October 2021–September 2022.
Methods
Study setting
This assessment was conducted in Kampala, the capital city of Uganda. It is divided into 5 administrative divisions: Central, Kawempe, Makindye, Rubaga, and Nakawa. The city has a surface area of 189 km2, including 176 km2 of land and 13 km2 of water [5]. The 2023 population was estimated at 1.76 million; however, the city has a dynamic and transient day population estimated at 5 million people [6]. Kampala Capital City Authority (KCCA) has been mandated to govern and administer Kampala Capital City on behalf of the Central Government of Uganda.
Kawempe National Referral Hospital (KNRH), located about 12km away from the city centre of Kampala, is a government-funded hospital largely providing free maternal and newborn healthcare services to patients referred from public and private health facilities within and outside Kampala City, as well as walk-ins from Kampala Metropolitan Area and surrounding districts. KNRH is serving a population of approximately 4.5 million [7]. The catchment area of the hospital has a heterogeneous population, consisting of the urban poor and those with average income. In 2019, 2,784 (11%) out of 24,526 deliveries were preterm based on KNRH records [8].
Study design, population, exclusion criteria, and sample size considerations
We conducted a retrospective cohort study among mothers who delivered at KNRH. Mothers with viable pregnancies of at least 28 weeks of gestation who delivered from the labour suite or theatre between October 1, 2021, and September 30, 2022, were identified to define our population-based birth cohort (n=22,192). Mothers whose weeks of gestation at birth or the first day of the last normal menstrual period were not documented in the file were excluded. Furthermore, all mothers whose first day of the last normal menstrual period occurred before October 1, 2021, were excluded to ensure that the exposure period accommodates all the weeks of gestation. Every mother with a valid telephone contact was reached out to confirm whether she resided in Kampala City, and then specify the division, parish, and duration of residence during pregnancy. Only 1,540 mothers who resided in Kampala City throughout their pregnancy time were enrolled in this assessment.
Study variables and data abstraction Covariates
We abstracted data regarding the covariates from the mother’s files using a data abstraction tool deployed in Kobo Collet. We considered the following potential individual covariates: demographic characteristics (maternal age, highest education level, marital status, occupation, HIV status), antenatal attendance, number of antenatal visits, history of smoking and alcohol use, and antepartum obstetric complications (eclampsia, pre-eclampsia, placenta abruptio, placenta previa, intra uterine fetal death (IUFD), preterm premature rupture of membranes, chorioamnionitis, and oligohydramnios). Only primary antepartum complications were considered for mothers who had multiple complications. Eclampsia and pre-eclampsia were categorized under hypertensive disorders. Placenta abruption and placenta previa were categorized under antepartum hemorrhage. Information on any covariates with missing data were obtained through telephone interviews with the mothers.
Gestational PM2.5 exposure
In December 2019, KCCA installed twenty–four Clarity© Node Solar–Powered monitors for outdoor air quality monitoring in all five divisions of Kampala City (Figure 1). Clarity© Node Solar–Powered monitors were permanently set up at least 1.5 meters above ground level in secure areas away from obstruction or emission sources that could interfere with air quality measurements. Calibration of Clarity© Node Solar–Powered monitors was based on co–location data with the reference air quality monitoring station at the US Embassy in Kampala City. Clarity© Node Solar–Powered monitors use inbuilt cellular connectivity to transmit raw data for PM2.5, PM10, nitrogen dioxide (NO2), temperature, and relative humidity. Calibrated data generated by these monitors are accessed in real–time on the Clarity© Dashboard by authorized KCCA staff and Clarity© operating team.
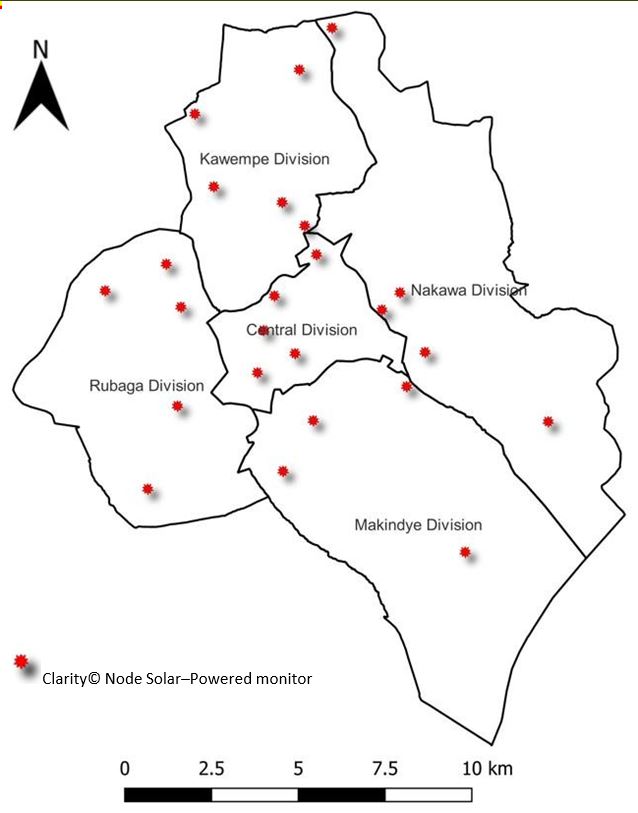
We abstracted 24–hour average PM2.5 concentrations generated by calibrated Clarity© Node Solar– Powered monitors from the Clarity© Dashboard from October 1, 2021, to September 30, 2022. For each mother, the centroid coordinates for the respective parish of residence in Kampala City was obtained from the geo-spatial database for Kampala City. The distance between parish coordinates and coordinates for all the Clarity Node Solar-Powered monitors installed in the city was calculated. The nearest Clarity Node Solar-Powered monitor to the parish of the mother was determined based on the least distance obtained. We estimated PM2.5 exposure during pregnancy based on average PM2.5 concentration obtained from the nearest Clarity© Node Solar–Powered monitor throughout the gestation period.
Outcome variable
Our outcome variable was preterm birth, defined as delivery before 37 weeks or 259 days from the first day of a pregnant woman’s last menstruation to delivery. Preterm birth was categorized into extremely very preterm birth (28 to <32 weeks) and moderate preterm birth (32 to <37 weeks) [9, 10]. Weeks of gestation were determined following standard obstetric practice based on first day of the last normal menstrual period or obtained from the ultrasound scan done during the first trimester for mothers who did not recall their first day of the last normal menstrual period.
Data analysis
Descriptive statistics were performed for demographic characteristics, antenatal attendance, and antepartum complications. We computed the incidence of preterm births (28<37 weeks) and further stratified into extremely very preterm birth (28 to <32 weeks) and moderate preterm birth (32 to <37 weeks). We calculated gestational PM2.5 exposure among pregnant mothers who experienced preterm birth (28<37 weeks) and term birth (≥37 weeks). We applied Mann Whitney U test to compare gestational PM2.5 exposure between pregnant mothers who experienced preterm birth and term birth. Gestational exposure to PM2.5 concentration was considered as the principal predictor of PTB.
Due to variability in the exposure length (weeks of gestation) for each birth, we utilized the Cox proportional hazards models based on weeks of gestation to determine the association between gestational PM2.5 exposure over the entire pregnancy and the risk of preterm birth. We subsequently adjusted for potential covariates using multivariate regression with the Cox proportional hazards model to obtain adjusted hazard ratios (HR), corresponding 95% confidence intervals and p-values. We stratified data by each covariate to generate stratum-specific hazard ratios between gestational PM2.5 exposure and preterm birth.
To assess whether antepartum complications modified the relationship between gestational PM2.5 concentration and preterm birth, we generated interaction terms between gestational PM2.5 concentration and antepartum complications. Interpretation of the interaction term’s hazard ratios and respective statistical significance explained whether the effect of gestational PM2.5 concentration on preterm birth varied by the presence or absence of the antepartum complications. We used the Wald’s Chi-squared test to assess the statistical significance of the multiplicative interaction between gestational PM2.5 exposure and antepartum complications.
Statistical significance was set at a p-value <0.05. All statistical analyses were performed using STATA 16.0.
Ethical considerations
The Office of the Associate Director for Science, US Centres of Disease Control and Prevention/Uganda, also determined that this activity was not human subject research, and its primary intent was public health practice or a disease control activity (specifically, epidemic or endemic disease control activity). Administrative clearance to extract data from patient files and PM2.5 exposure data from the Clarity© Dashboard was obtained from Kawempe National Referral Hospital and Kampala Capital City Authority respectively. All methods were performed in accordance with the approval and administrative clearance. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy. §
- See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Results
Characteristics of study participants during an evaluation of gestational PM2.5 exposure on preterm births in Kampala City, Uganda, October 2021–September 2022
Out of 1,540 pregnant mothers, 1,030 (66.9%) were aged 18–29 and 892 (57.9%) were housewives. Of note, only 819 (53.2%) attended antenatal care. Only 382 (24.8%) had antenatal obstetric complications (Table 1).
Table 1: Characteristics of study participants during an evaluation of gestational PM2.5 exposure on preterm births in Kampala City, Uganda, October 2021–September 2022
| Study variables | Frequencies
(n=1,540) |
Percentages (%) |
| Completed age | ||
| Below 18 | 53 | 3.4 |
| 18–29 | 1,030 | 66.9 |
| 30–39 | 419 | 27.2 |
| 40–49 | 38 | 2.5 |
| Division of residence | ||
| Kawempe | 466 | 30.3 |
| Makindye | 336 | 21.8 |
| Nakawa | 105 | 6.8 |
| Rubaga | 428 | 27.8 |
| Central | 205 | 13.3 |
| Highest education level | ||
| None | 412 | 26.8 |
| Primary | 346 | 22.5 |
| Secondary | 515 | 33.4 |
| Tertiary or above | 267 | 17.3 |
| Marital Status | ||
| Not Married | 236 | 15.3 |
| Married | 1,304 | 84.7 |
| Occupation | ||
| House wife | 892 | 57.9 |
| Formal employment | 156 | 10.1 |
| Business | 492 | 32.0 |
| Number of children | ||
| 1 – 2 | 931 | 60.5 |
| 3 – 4 | 447 | 29.0 |
| 5 and above | 162 | 10.5 |
| HIV status | ||
| Negative | 1,412 | 91.7 |
| Positive | 107 | 6.9 |
| Unknown | 21 | 1.4 |
| Antenatal attendance | ||
| No | 721 | 46.8 |
| Yes | 819 | 53.2 |
| Number of antenatal visits (n=819) | ||
| 1 – 2 | 111 | 13.6 |
| 3 – 4 | 401 | 48.9 |
| 5 and above | 307 | 37.5 |
| History of smoking | ||
| No | 1,523 | 98.9 |
| Yes | 17 | 1.1 |
| History of alcohol use | ||
| No | 1,512 | 98.2 |
| Yes | 28 | 1.8 |
| Antenatal obstetric complication | ||
| No | 1,158 | 75.2 |
| Yes | 382 | 24.8 |
| Preterm premature rupture of membranes | ||
| No | 1,436 | 93.2 |
| Yes | 104 | 6.8 |
| Hypertensive disorder | ||
| No | 1,431 | 92.9 |
| Yes | 109 | 7.1 |
| Antepartum hemorrhage | ||
| No | 1,484 | 96.4 |
| Yes | 56 | 3.6 |
| Oligohydramnios | ||
| No | 1,495 | 97.1 |
| Yes | 45 | 2.9 |
| Chorioamnionitis | ||
| No | 1,493 | 97.0 |
| Yes | 47 | 3.0 |
| Intra uterine fetal death (IUFD) | ||
| No | 1,519 | 98.6 |
| Yes | 21 | 1.4 |
Incidence of preterm births in Kampala City, Uganda, October 2021–September 2022
Among pregnant mothers who resided in Kampala City throughout pregnancy and delivered from KNRH, 229 (14.9%) had preterm births (Table 2). Of these, the majority, 198 (86.5%) had moderate to late preterm births whereas only 31 (13.5%) had very preterm births.
Gestational PM2.5 exposure
Overall, the average gestational PM2.5 exposure was 66μg/m3 (range: 45–75μg/m3). Gestational PM2.5 exposure was slightly higher among pregnant mothers who experienced preterm birth compared to those who delivered at term (Table 2). Significant difference in gestational PM2.5 exposure was observed between pregnant mothers who experienced preterm birth and those who delivered at term (p=0.002).
Factors associated with incidence of preterm births in Kampala City, Uganda, October 2021– September 2022
At multivariate analysis, gestational PM2.5 exposure and development of hypertensive disorders were statistically significantly associated with incidence of preterm births among pregnant mothers in Kampala City. For every unit increase in average PM2.5 exposure during pregnancy, the risk of incidence of a preterm birth increases by 3% (HR=1.03 [1.01 – 1.05]). Pregnant mothers who developed hypertensive disorders were 61% more likely to have preterm births compared to those who did not develop hypertensive disorders during pregnancy (HR=1.61 [1.05 – 2.48]) (Table 2).
Table 2: Factors associated with incidence of preterm births in Kampala City, Uganda, October 2021–September 2022
| Study variables | Term birth n (%) | Preterm birth n (%) | Adjusted hazard ratios | 95% confidence intervals |
| Socio-demographic characteristics | ||||
| Completed age | ||||
| Below 18 | 43 (3.3) | 10 (4.4) | Ref. | |
| 18–29 | 879 (67.0) | 151 (65.9) | 0.63 | [0.33 – 1.21] |
| 30–39 | 358 (27.3) | 61 (26.6) | 0.59 | [0.29 – 1.17] |
| 40–49 | 31 (2.4) | 7 (3.1) | 0.79 | [0.30 – 2.09] |
| Highest education level | ||||
| None | 348 (26.5) | 64 (27.9) | Ref. | |
| Primary | 297 (22.7) | 49 (21.4) | 0.86 | [0.58 – 1.25] |
| Secondary | 440 (33.6) | 75 (32.8) | 0.91 | [0.64 – 1.28] |
| Tertiary or above | 226 (17.2) | 41 (17.9) | 0.92 | [0.61 – 1.40] |
| Marital Status | ||||
| Married | 1,118 (85.3) | 186 (81.2) | Ref. | |
| Not married | 193 (14.7) | 43 (18.8) | 1.31 | [0.92 – 1.86] |
| Occupation | ||||
| House wife | 761 (58.0) | 131 (57.2) | Ref. | |
| Business | 423 (32.3) | 69 (30.1) | 0.93 | [0.69 – 1.26] |
| Formal employment | 127 (9.7) | 29 (12.7) | 1.21 | [0.79 – 1.86] |
| HIV status | ||||
| Negative | 1,210 (92.3) | 202 (88.2) | Ref. | |
| Positive | 86 (6.6) | 21 (9.2) | 1.31 | [0.83 – 2.08] |
| Unknown | 15 (1.1) | 6 (2.6) | 1.82 | [0.77 – 4.26] |
| Antenatal attendance | ||||
| No | 624 (47.6) | 97 (42.4) | Ref. | |
| Yes | 687 (52.4) | 132 (57.6) | 1.13 | [0.85 – 1.49] |
| History of smoking | ||||
| No | 1,297 (98.9) | 226 (98.7) | Ref. | |
| Yes | 14 (1.1) | 3 (1.3) | 0.93 | [0.29 – 2.98] |
| History of alcohol use | ||||
| No | 1,289 (98.3) | 223 (97.4) | Ref. | |
| Yes | 22 (1.7) | 6 (2.6) | 1.20 | [0.52 – 2.79] |
| Gestational PM2.5 exposure [Mean (range)] | ||||
| PM2.5 concentration | 66 (45 – 75) | 67 (53 – 75) | 1.03 | [1.01 – 1.05] ** |
| Antepartum complications | ||||
| Preterm premature rupture of membranes | ||||
| No | 1,223 (93.3) | 213 (93.0) | Ref. | |
| Yes | 88 (6.7) | 16 (7.0) | 1.24 | [0.73 – 2.08] |
| Hypertensive disorder | ||||
| No | 1,221 (93.6) | 204 (89.1) | Ref. | |
| Yes | 84 (6.4) | 25 (10.9) | 1.61 | [1.05 – 2.48] * |
| Antepartum hemorrhage | ||||
| No | 1,264 (96.4) | 220 (96.1) | Ref. | |
| Yes | 47 (3.6) | 9 (3.9) | 1.23 | [0.62 – 2.43] |
| Oligohydramnios | ||||
| No | 1,274 (97.2) | 221 (96.5) | Ref. | |
| Yes | 37 (2.8) | 8 (3.5) | 1.37 | [0.68 – 2.82] |
| Chorioamnionitis | ||||
| No | 1,271 (96.9) | 222 (96.9) | Ref. | |
| Yes | 40 (3.1) | 7 (3.1) | 1.06 | [0.49 – 2.27] |
| Intrauterine fetal death | ||||
| No | 1,296 (98.9) | 223 (97.4) | Ref. | |
| Yes | 15 (1.1) | 6 (2.6) | 1.87 | [0.81 – 4.29] |
* p<0.05 ** p<0.01 *** p<0.001
Association between gestational PM2.5 exposure and preterm birth stratified by antepartum complications
There was a significant risk of preterm birth attributed to gestational PM2.5 exposure among mothers without preterm premature rupture of membranes, hypertensive disorders, antepartum hemorrhage, oligohydramnios, chorioamnionitis, and intra uterine fetal deaths compared to those with these antepartum complications. Based on p-values, there was no statistically significant difference in stratum specific hazard ratios between gestational PM2.5 exposure and incidence of preterm birth by antepartum complications (Table 3).
Table 3: Association between gestational PM2.5 exposure and preterm birth stratified by antepartum complications, in Kampala City, Uganda, October 2021–September 2022
| Antepartum complication | Frequency (%) | Stratum specific hazard ratios1 | 95% Confidence Intervals | p-value | |
| Preterm premature rupture of membranes | 0.696 | ||||
| No | 1,436 (93.2) | 1.04 | [1.01 – 1.06] ** | ||
| Yes | 104 (6.8) | 1.00 | [0.93 – 1.09] | ||
| Hypertensive disorder | 0.564 | ||||
| No | 1,431 (92.9) | 1.03 | [1.01 – 1.06] ** | ||
| Yes | 109 (7.1) | 1.00 | [0.93 – 1.09] | ||
| Antepartum hemorrhage | 0.511 | ||||
| No | 1,484 (96.4) | 1.03 | [1.00 – 1.05] ** | ||
| Yes | 56 (3.6) | 1.10 | [0.93 – 1.31] | ||
| Oligohydramnios | 0.897 | ||||
| No | 1,495 (97.1) | 1.03 | [1.01 – 1.06] ** | ||
| Yes | 45 (2.9) | 1.09 | [0.92 – 1.30] | ||
| Chorioamnionitis | 0.518 | ||||
| No | 1,493 (97.0) | 1.03 | [1.01 – 1.06] ** | ||
| Yes | 47 (3.0) | 1.33 | [0.92 – 1.93] | ||
| Intra uterine fetal death | 0.098 | ||||
| No | 1,519 (98.6) | 1.03 | [1.01 – 1.06] ** | ||
| Yes | 21 (1.4) | 0.97 | [0.87 – 1.09] | ||
* p<0.05 ** p<0.01 *** p<0.001
1Adjusted for age, educational level, marital status, occupation, HIV status, antenatal attendance, history of smoking and alcohol use @p-values for Wald’s Chi-squared test for interaction (p-values for effect modification)
Discussion
In our assessment, gestational exposure to PM2.5 concentration was considered the primary predictor of preterm birth (PTB) in Kampala City, and we subsequently adjusted for potential covariates using multivariate regression with the Cox proportional hazards model. We observed a 3% rise in the risk of PTB for each incremental unit increase in average gestational PM2.5 exposure (HR=1.03, 95% CI: 1.01–1.05), after adjusting for potential covariates in our multivariate regression analysis. Notably, pregnant mothers who developed hypertensive disorders exhibited a higher likelihood of experiencing preterm births compared to their counterparts. Despite the well-established elevated risk of preterm births associated with gestational PM2.5 exposure among mothers without preterm premature rupture of membranes, hypertensive disorders, antepartum hemorrhage, oligohydramnios, chorioamnionitis, and intrauterine fetal deaths, we found that the effect modification by these antepartum complications did not achieve statistical significance.
Our findings align with previous studies which have demonstrated the increased risk of preterm births attributed to gestational PM2.5 exposure. A study in Italy observed a 3% risk of preterm births per unit increase in gestational PM2.5 exposure [11]. A multi country study in Africa reported a significant association between gestational PM2.5 exposure and increased risk of preterm births [1.08 (95% CI: 1.01–1.16)] [3]. A significant risk of preterm birth estimated at 1.2% per unit increase in gestational PM2.5 exposure was reported based meta regression analysis across 204 countries [12]. However, inconsistent findings were reported in a retrospective cohort study among 2,966,705 million singleton live births in Canada, where gestational PM2.5 exposure provided a protective effect by reducing the risk of preterm births by 20% [0.80 (95% CI: 0.75–0.86)] [13].
Another retrospective cohort study among 7,961 births occurring from June 2008 to May 2010 in Detroit, Michigan, United States revealed that maternal exposure to PM2.5 was not statistically significantly associated with incidence of preterm birth (p value = 0.376) [14]. Differences in air quality, population characteristics, sample size, time periods, and PM2.5 exposure measurement among studies conducted in different regions, such as Kampala, Canada and Detroit, may explain variations in the observed effects of gestational PM2.5 exposure on preterm birth, with one study suggesting a protective effect while another found no significant association. Nevertheless, the significant association between gestational PM2.5 exposure and preterm birth indicates the effect of air pollution on health outcomes. Such findings should implore relevant stakeholders to prioritize implementation of appropriate interventions to avert anticipated health consequences in Kampala City, where the 24–hour average PM2.5 concentration from January, 2020–June, 2022, was 59 µg/m3; exceeding targeted WHO targeted safe levels [15].
The increased risk of preterm birth among pregnant mothers who developed hypertensive disorders has been previously evidenced. A recent scoping review highlighted hypertensive disorders among the most frequently reported risk factors for preterm births in Sub Saharan Africa [16]. A meta-analysis conducted in East African countries reported that mothers who had pregnancy induced hypertension were three times likely to experience preterm births compared to their counterparts [17]. What is quite challenging is that the risk of developing hypertensive disorders during pregnancy has also been attributed to gestational PM2.5 exposure. Gestational PM2.5 exposure increases the risk of pregnancy induced hypertensive disorders following endothelial dysfunction, autonomic nervous system imbalance, oxidative stress, and systemic inflammatory response [18-20]. A 5 µg/m3 increment in gestational PM2.5 exposure significantly increased the risk of developing pregnancy-induced hypertensive disorders by 57% [21]. Due to the complex relationship of gestational PM2.5 exposure, hypertensive disorders, and preterm birth, the observed risk of preterm births among pregnant mothers who developed hypertensive disorders may as well be indirectly confounded by gestational PM2.5 exposure.
Despite the growing evidence on the association between air pollution and preterm births, very few studies have investigated whether the association between gestational PM2.5 exposure and birth outcomes vary by antepartum complications. We hypothesized that the effect of gestational PM2.5 exposure on incidence of preterm births might be modified by antepartum complications such as preterm premature rupture of membranes, hypertensive disorders, antepartum hemorrhage, oligohydramnios, chorioamnionitis and intra uterine fetal deaths. However, this was not evidenced in this study with reference to the observed non-significant effect modification. The observed non- significant effect modification by hypertensive disorders was synonymous with findings from a population based cohort study in California which concluded that pre-existing and pregnancy-induced hypertension did not modify the relationship between air pollution and preterm birth [22]. This implies that the effect of gestational PM2.5 exposure on incidence of preterm births does not differ depending on the presence or absence of the antepartum complication.
Study strengths and limitations
Our findings should be interpreted in line with the following limitation. We were unable to reach out to 1,354 mothers who met the eligibility criteria because they did not have valid documented telephone contacts. We were not able to confirm their primary address and thus excluded them from the analysis. This could have led to underestimation or over estimation of the risk of preterm births attributed to gestational PM2.5 exposure in Kampala City. Despite the limitation, this assessment presents the first comprehensive investigation examining the association between gestational PM2.5 exposure and preterm birth in Kampala City using ground-level air quality data sourced from Clarity© Node Solar-Powered low-cost monitors.
Conclusion
We observed a significant impact of air pollution on the incidence of preterm births in Kampala City. Kampala Capital City Authority and other relevant partners are implored to prioritize interventions aimed at reducing air pollution to improve maternal and child health. Furthermore, efforts aimed at reducing preterm births should not underscore the urgent need to mitigate air pollution.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contributions
MN designed the protocol under the technical guidance of AN, AO, SZ, DA, TK, PW, LA, DK, LB, RM, ARA, LA, JRH, and DAO. MN, AN, DA, TK and SZ supervised data collection, analyzed, and interpreted the data. MN drafted the bulletin. MN, AN, AO, PW, LA, DK, LB, RM, ARA, LA, JRH, and DAO, critically reviewed the bulletin for intellectual content. All co–authors read and approved the final bulletin.
Acknowledgments
We acknowledge the administration of KNRH for the administrative clearance to review mother’s files and collect secondary data required for the execution of this project. Additional thanks to Clarity© Movement Company and Department of Public Health and Environment, KCCA for providing access to air quality data generated by Clarity© Node Solar–Powered monitors. Ultimately, the authors would like to thank research officers: Merab Nabaasa and Happiness Tusimiirwe who coordinated patient data collection in Kawempe National Referral Hospital.
Copyright and licensing
All materials in the Uganda Public Health Bulletin is in the public domain and may be used and re- printed without permission; citation as to source; however, is appreciated. Any article can be re- printed or published. If cited as a reprint, it should be referenced in the original form.
References
- Zhang, L., et al., Short-term and long-term effects of PM2. 5 on acute nasopharyngitis in 10 communities of Guangdong, Science of the Total Environment, 2019. 688: p. 136-142.
- Malley, S., et al., Preterm birth associated with maternal fine particulate matter exposure: a global, regional and national assessment. Environment international, 2017. 101: p. 173-182.
- Bachwenkizi, , et al., Maternal exposure to fine particulate matter and preterm birth and low birth weight in Africa. Environment International, 2022. 160: p. 107053.
- IQAir, 2021 World Air Quality Report: Region & City PM2.5 Ranking. Available from: file:///C:/Users/HP/Downloads/world-air-quality-report-2021-en%20(1).pdf. Accessed on: 9th June 2021.
- Alex, N., et al., A community perspective of flood occurrence and weather forecasting over Kampala City. African Journal of Environmental Science and Technology, 2021. 15(5): 188- 201.
- UBOS, Population Projections 2015 to Accessed on: May 10, 2023. Available from: https://www.ubos.org/population-projections-2015-to-2030. 2015.
- Serugo, G., Horror at Kawempe hospital. Accessed on: September 15, 2023. Available from: https://observer.ug/news/headlines/74786-horror-at-kawempe-hospital, in The Observer. 2022: Kampala,
- Kayiga, H., et al., Incidence, associated risk factors, and the ideal mode of delivery following preterm labour between 24 to 28 weeks of gestation in a low resource Plos one, 2021. 16(7): p. e0254801.
- Blencowe, , et al., Born too soon: the global epidemiology of 15 million preterm births. Reproductive health, 2013. 10(1): p. 1-14.
- Quinn, -A., et al., Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine, 2016. 34(49): p. 6047-6056.
- Ottone, M., et al., Source-related components of fine particulate matter and risk of adverse birth outcomes in Northern Environmental Research, 2020. 186: p. 109564.
- Ghosh, , et al., Ambient and household PM2. 5 pollution and adverse perinatal outcomes: A meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS medicine, 2021. 18(9): p. e1003718.
- Stieb, M., et al., Associations of pregnancy outcomes and PM2. 5 in a national Canadian study. Environmental health perspectives, 2016. 124(2): p. 243-249.
- Cassidy-Bushrow, A.E., et al., Prenatal airshed pollutants and preterm birth in an observational birth cohort study in Detroit, Michigan, Environmental research, 2020. 189: p. 109845.
- Ninsiima, , et al., Spatio–temporal trends of air quality, Kampala City, Uganda, 2020–2022. 2023.
- Mabrouk, , et al., A Scoping Review of Preterm Births in Sub-Saharan Africa: Burden, Risk Factors and Outcomes. International journal of environmental research and public health, 2022. 19(17): p. 10537.
- Laelago, , T. Yohannes, and G. Tsige, Determinants of preterm birth among mothers who gave birth in East Africa: systematic review and meta-analysis. Italian Journal of Pediatrics, 2020. 46(1): p. 10.
- Brook, D., et al., Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension, 2009. 54(3): p. 659-667.
- Sun, Q., et al., Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal Jama, 2005. 294(23): p. 3003-3010.
- Sun, Q., et al., Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arteriosclerosis, thrombosis, and vascular biology, 28(10): p. 1760-1766.
- Pedersen, , et al., Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension, 2014. 64(3): p. 494-500.
- Weber, A., et al., Air pollution, maternal hypertensive disorders, and preterm birth. Environmental Epidemiology, 2019. 3(5).


Comments are closed.