Assessment of adverse events following AstraZeneca COVID-19 vaccination in Greater Kampala, Uganda, March-April, 2021
Authors: Allan Komakech1*, John Kamulegeya2, Freda Aceng3, Jonathan Izudi4, James Acaye5, Edirisa Junior Nsubuga1, Petranilla Nakamya1, Benon Kwesiga1, Daniel Kadobera1, Lilian Bulage1, Alex Riolexus Ario1 Institutional Affiliations 1Uganda Public Health Fellowship Program Uganda National Institute of Public Health Kampala, Uganda, 2World Health Organization, Kampala, Uganda, 3Department of Integrated Epidemiology, Surveillance and Public Health Emergencies, Ministry of Health, Kampala, Uganda, 4Clarke International University, Kampala, Uganda, 5Mulago Hospital, Kampala, Uganda. *Correspondence: Tel: +256789185617, Email: akomackech@musph.ac.ug
Summary
Background: Tracking of adverse events following vaccination is important for evaluating vaccine safety. During March 2021, Uganda began COVID-19 vaccination using the Astra-Zeneca vaccine targeting teachers, health workers, security personnel and the elderly. We assessed adverse events following AstraZeneca vaccination in Greater Kampala, Uganda to evaluate the safety of the vaccine.
Methods: We used vaccination registers to identify persons who received ≥1 dose of the AstraZeneca COVID-19 vaccine during March 10–April 30, 2021. Adverse events following vaccination were defined as an untoward medical occurrence after vaccination (not necessarily causally related to the vaccine). Serious adverse events were defined as any event considered life-threatening, resulting in in-patient hospitalization, persistent disability ˃28 days, death or congenital anomaly. We extracted telephone contacts for a systematic random sample of vaccinated individuals and their next of kin where necessary. We then conducted phone interviews with those who consented to collect data on demographics and details of adverse events where they occurred.
Results: Among 374 participants interviewed, mean age was 41 years SD 13 years; range 20 – 85 years; 176 (47%) were female. Of these, 235 (63%) received only one dose and 139 (37%) received two doses. Among the participants, 286 (77%) reported at least one adverse event. Of these, 255 (68%) reported the occurrence of adverse events after receiving the first dose whereas 45 (32%) encountered adverse events after receiving the second dose. The most common adverse events were redness/pain/itching at injection site (34%) and headache (32%). None of the events were classified as serious. Persons aged 20–29 years (AOR 4.7; 95% CI: 2.0–10.2), 30-39 years (AOR 3.7; 95% CI: 1.8–7.4) and 40-49 years (AOR 2.8; 95% CI 1.3–5.0) were more likely to develop adverse events compared to those aged ≥50 years.
Conclusion: Most individuals experienced ≥1 adverse event. No serious adverse events were reported. We recommend administration of the AstraZeneca COVID-19 vaccine in Uganda based on its safety.
Introduction
On March 11, 2020, the World Health Organization (WHO) declared the Coronavirus Disease 2019 (COVID-19) pandemic (1). COVID-19 is an illness caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (1,2). COVID-19 can manifest as an asymptomatic, mild, moderate or severe illness and can result in death (1,3). The most common symptoms of COVID-19 include fever, cough, fatigue, and dyspnea (4). To slow the spread of SARS-CoV-2, nations around the world implemented different control measures. These measures included social distancing, partial and comprehensive lockdowns, closing of schools and businesses, and wearing face masks in public (5). Although such measures helped in flattening the epidemic curve, the resurgence of COVID-19 was reported as societies and economies reopened (6). Hence, there was an urgent need for vaccination as a long-term preventive measure to address COVID-19 (7).
In November 2020, the first vaccines to protect against the severity of COVID-19 were approved (8). Since then, several vaccines have been rolled out in countries, and more than 200 additional vaccine candidates are still in development (9). The most commonly used vaccines include the AstraZeneca, Moderna, BioNTech, Pfizer, Johnson & Johnson, Sinopharm, and Gamaleya vaccines (10). Following vaccination, adverse events can occur within seconds to weeks (11). Adverse events are events that occur after vaccination but may not necessarily have a causal relationship with usage of the vaccine (11,12). Adverse events can be categorized as serious and non-serious. Serious adverse events are events that result in death, hospitalization, a permanent/persistent disability or congenital anomalies/defects. Non-serious adverse events are events that do not meet the criteria for serious events (13,14).
In March 2021, the Uganda Ministry of Health (MoH) started the vaccination exercise using the AstraZeneca COVID-19 vaccine, and distribution to all districts was done (15). The target group for vaccination included: teachers, health workers, security personnel, humanitarian front-line workers, the elderly (≥50 years) and those aged 18–49 years with comorbidities (16). In order to track adverse events, the MoH instituted active surveillance for adverse events as recommended by the World Health Organization (WHO) in the post-authorization/Emergency Use Listing period (17). Tracking of adverse events following vaccination is important for evaluating the safety of vaccines.
Expected adverse events for the AstraZeneca COVID-19 vaccine included injection site events, headaches, fever and malaise among others (18). With the AstraZeneca vaccine in particular, concern were raised by scientists in Europe about serious adverse events such as deaths, clots, and severe allergic reactions (19). Although the Uganda MoH and WHO assured the public that the AstraZeneca vaccine is safe and effective against COVID-19 (20), no study had been done to fully evaluate adverse events experienced with the vaccine in Uganda. We described the adverse events experienced following AstraZeneca COVID-19 vaccination and determined the factors associated with the occurrence of adverse events in Greater Kampala, Uganda.
Methods and materials
Case definition
We defined a serious adverse event as any medical occurrence that resulted in hospitalization or was considered life-threatening in an individual who received at least one dose of the AstraZeneca COVID-19 vaccine from a vaccination site in Greater Kampala, Uganda during March 10–April 30, 2021. A non-serious adverse event was defined as one that did not meet the criteria for serious adverse events.
Study design
We conducted a cross-sectional study on the experiences of people who received at least one dose of the COVID-19 vaccine. Quantitative methods were employed.
Study area
We conducted the study in Kampala, Wakiso, and Mukono districts, districts that make up Greater Kampala. These districts were chosen because they had the highest proportion of individuals who had received the COVID-19 vaccine at the time, with Kampala (15%), Mukono (2%) and Wakiso (1.7%). Early during the vaccination exercise, the Uganda MoH gazetted COVID-19 vaccination sites in each district. In Kampala Capital City, five vaccination sites were designated for COVID–19 vaccination in each of the five divisions. During the same period, only five vaccination sites per district had been gazetted in Wakiso and Mukono districts.
Sample size
All the 35 vaccination sites located within the Greater Kampala were considered for the study. The sample size for the participants was determined using the Kish Leslie (1964) formula, assuming a 95% confidence Interval, 50% estimated incidence of adverse events, and a margin of error (precision) of 0.05. Based on these assumptions, we estimated that we would need to include 384 individuals for which we inflated this number by 30% to account for non-response.
Sampling procedure
For each of the vaccination sites, we established the proportions of participants who would qualify for the study using probability proportionate to size of vaccinated individuals. At the time of data collection, approximately 36,000 individuals had received the AstraZeneca vaccine in Kampala, Wakiso and Mukono. We apportioned our sample size among the 35 vaccination sites based on the number who had received the vaccine at each of the vaccination sites. We selected 355 participants from 25 vaccination sites in Kampala District, 47 participants from five vaccination sites in Mukono district and 40 participants from five vaccination sites in Wakiso District. We then applied systematic sampling with a random start to identify the individuals who qualified for the study. At each vaccination site, a list of vaccinated individuals was generated from the vaccination registers. We established a sampling interval (i) by dividing the total number of vaccinated individuals at the vaccination site by the required sample size per vaccination site. We then selected a random number (r) between 1 and the i value as our starting point. Individuals (r, r+i, r+2i etc) were selected to participate in our study. We extracted phone numbers of the selected participants from the vaccination registers.
Study variables and data collection
We used a standardized questionnaire adapted from the WHO core variables for adverse events . The primary outcome variable for this study was experiencing an adverse event following vaccination that could occur within seconds to weeks after vaccination. We collected data on demographic characteristics (age, sex, nationality, profession), individual clinical characteristics (having a chronic illness, ever had previous reactions to a vaccine, usually have reactions to medicine and illness at the time they received the vaccine) and details of where the adverse events occurred. Data collected on adverse events included: adverse events experienced, the dose after which they occurred, time of onset, duration, and outcome of the adverse events. Information from eligible participants was collected through phone interviews. For participants and their next of kin whose phone numbers were not reachable at the first attempt to have an interview, we scheduled up to three other attempts to contact them at later dates.
Data analysis
For quantitative data analysis, we entered data on demographics, individual clinical characteristics, and details of where adverse events occurred into an excel abstraction spreadsheet and analyzed this data using SPSS. Frequencies and percentages were presented for all the categorical variables. Binary logistic regression model was fitted to establish the association between the categorical independent variables and the outcome of interest (experiencing an adverse event following vaccination). First, crude odds ratios, 95% confidence interval (CI) and their respective p-values were obtained. All variables with p-values <0.10 were considered for multivariable analysis. Multivariable logistic regression was conducted to obtain adjusted odds ratios with their respective 95% confidence interval (CI) and p-values. Variables with p-values <0.05 at multivariable analysis were considered to be significant and associated with the outcome.
Results
Demographic and clinical characteristics of study participants during a study assessing adverse events following AstraZeneca COVID – 19 vaccination in Greater Kampala, Uganda, March–April 2021
A total of 374 participants were interviewed. The mean age of the participants was 41 years (range 20 – 85), SD 13 years. Of the participants, 352 (94%) reported that they didn’t have any illness at the time of receiving the COVID – 19 vaccine as shown in Table 1.
Table 1: Demographic and clinical characteristics of study participants
| Variables | Frequency, n | Percentage (%) |
| Health facility/vaccination site | ||
| Kampala | 280 | 75 |
| Mukono | 56 | 15 |
| Wakiso | 38 | 10 |
| Age group (years) | ||
| 20–29 | 73 | 20 |
| 30–39 | 113 | 30 |
| 40–49 | 90 | 24 |
| Sex | 98 | 26 |
| Male | 198 | 53 |
| Female | 176 | 47 |
| Nationality | ||
| Ugandan | 365 | 98 |
| Non–Ugandan | 9 | 2.4 |
| Profession | ||
| Health workers | 67 | 18 |
| Teacher | 83 | 22 |
| Security officers | 11 | 3 |
| Others | 213 | 57 |
| Have chronic illness | ||
| Yes | 104 | 28 |
| No | 270 | 72 |
| Currently on any long-term medication | ||
| Yes | 70 | 19 |
| No | 304 | 81 |
| Ever had previous reactions to vaccinations | ||
| Yes | 19 | 5 |
| No | 330 | 88 |
| Not sure | 25 | 7 |
| Usually have reactions to any medicine such as antibiotics, anti-inflammatories | ||
| Yes | 47 | 13 |
| No | 327 | 87 |
| Illness at the time you received the COVID-19 vaccine | ||
| Yes | 22 | 6 |
| No | 352 | 94 |
Proportion of individuals that experienced adverse events following AstraZeneca COVID-19 vaccination
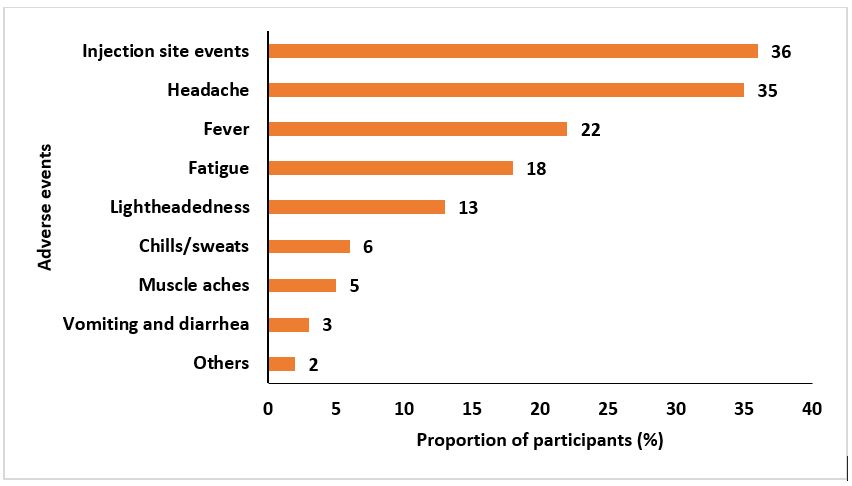
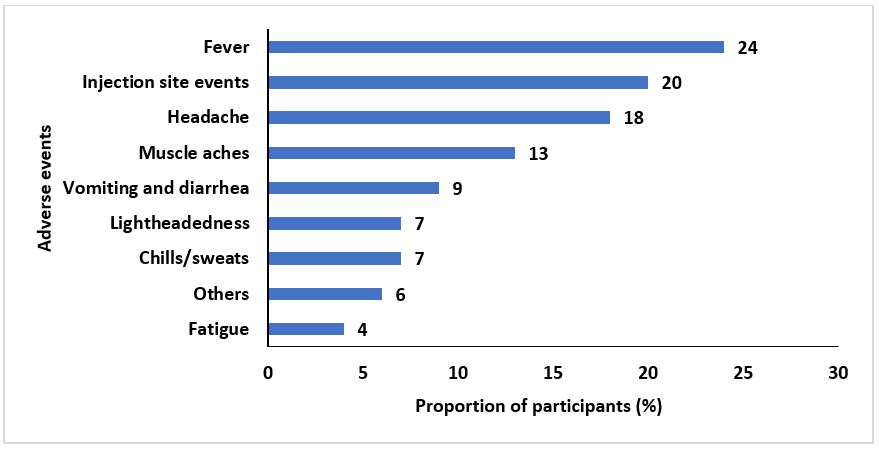
Among the participants, 255 (68%) experienced at least one event after the first dose and 45 (32%) experienced at least one event after the second dose; 268 (77%) had some form of adverse event after either dose. The most commonly reported adverse events following the first dose were: redness/pain/itching at injection site, 92 (36%); headache, 88 (35%); and fever, 56 (22%) (Figure 1). The most commonly reported adverse events following the second dose were: fever, 11 (24%); redness/pain/itching at injection site, 9 (20%); and headache, 8 (18%) (Figure 2). Overall, injection site events 101 (34%) and headache 96 (32%) were the most experienced adverse events. In the “Others” category, events such as skin rash, insomnia, limb paresis (mild/partial paralysis) and limb paraesthesia (burning or tingling sensation) were reported.
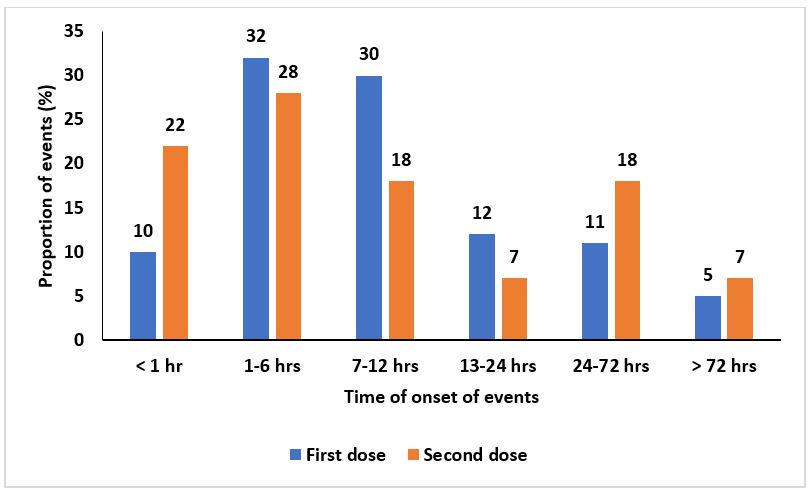
Time of onset of adverse events following AstraZeneca COVID-19 vaccination
Most adverse events 146 (32%) after the first dose and most adverse events after the second dose 17 (28%) commenced within 1-6 hours (Figure 3). Generally, most adverse events after the first and second dose commenced within the first 72 hours, 434 (95%) and 56 (93%) respectively (Figure 4). Furthermore, 23 (5%) and 4 (7%) of the adverse events commenced after 72 hours of receiving the first and second dose respectively as indicated in Figure 3.
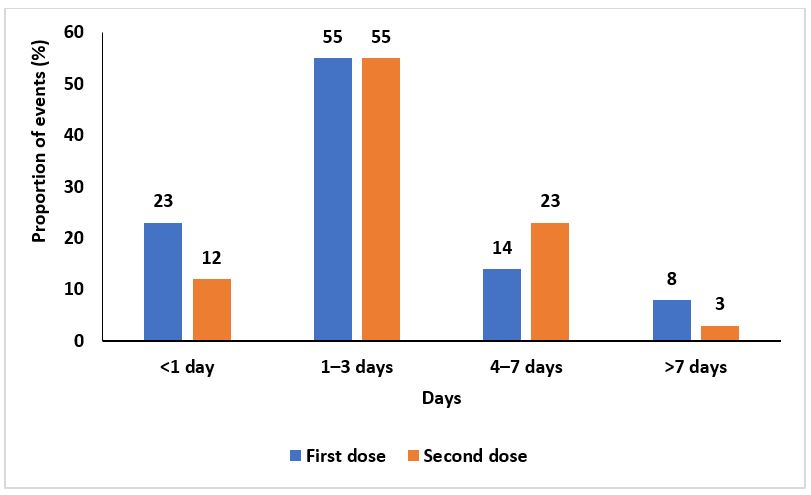
Duration of adverse events following AstraZeneca COVID – 19 vaccination
Two hundred fifty-two (55%) of the events after the first dose and 33 (55%) of the events after the second dose lasted for 1-3 days. Thirty-six (8%) of the events after the first dose and 6 (10%) of the events after the second dose lasted ˃7 days (Figure 4).
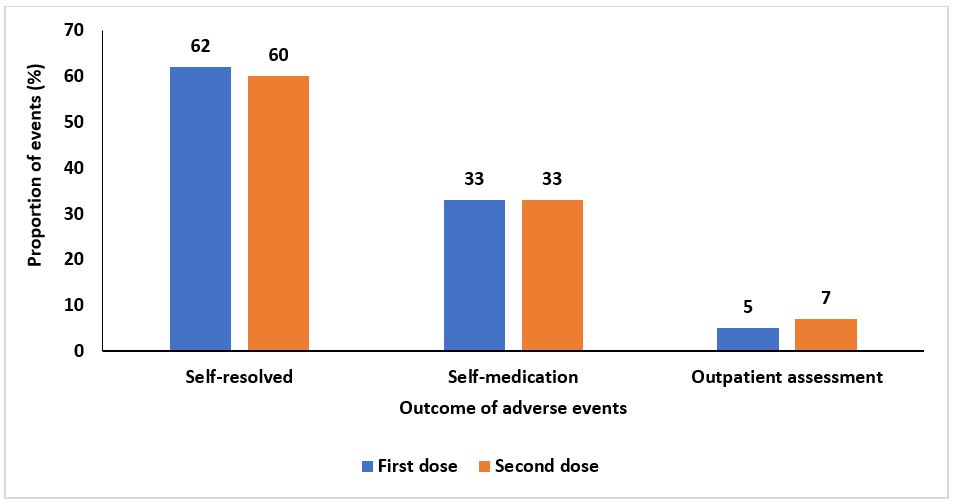
Two hundred eighty-two (62%) events after the first dose and 36 (60%) events after the second dose were self-resolved. Twenty-four (5%) of the events after the first dose and 4 (7%) of the events after the second dose resulted in outpatient assessment (Figure 5).
Factors associated with experiencing adverse events following AstraZeneca COVID – 19 vaccination (AEFIs) in Greater Kampala, Uganda, March–April 2021
Table 2 shows unadjusted and adjusted prevalence ratios with their respective corresponding confidence intervals. At bivariate analysis, age, sex, and currently being on long-term medication were associated with an adverse event at bivariate analysis. Respondents who were aged 20-29 years (COR 5.3, 95% CI, 2.4-12), 30-39 years (COR 3.9, 95% CI 2.1-7.5) and 40-49 years (COR 2.8, 95% CI, 1.5-5.3) were more likely to develop an adverse event than respondents aged 50 years and above. Females were more likely to develop an adverse event compared to males (COR 1.7, 95% CI, 1.02-2.7); and those who were not on long term medication were less likely to develop an adverse event than those who were on long term medication (COR 0.4, 95% CI, 0.2-0.8).
At multivariate analysis, age was significantly associated with developing an adverse event. Participants aged 20-29 years (AOR 4.7; 95% CI: 2.0–10.2), 30-39 years (AOR 3.7; 95% CI: 1.8–7.4) and 40-49 years (AOR 2.8; 95% CI 1.3–5.0) were more likely to develop an adverse event compared to those above 50 years old. Odds of developing an adverse event increased with decreasing age as shown in Table 2.
Table 2: Factors associated with adverse events following AstraZeneca COVID-19 vaccination
| Variables | Adverse event, n (%) | No adverse event, n (%) | COR (95% CI) | AOR (95% CI) |
| Age group (years) | ||||
| ≥50 | 56 (20) | 42 (48) | 1.0 | 1.0 |
| 20–29 | 64 (22) | 9 (10) | 5.3 (2.4–11.9) | 4.7 (2.0–10.2)** |
| 30–39 | 95 (33) | 18 (21) | 3.9 (2.1–7.5) | 3.7 (1.8–7.4)*** |
| 40–49 | 71 (25) | 19 (22) | 2.8 (1.5–5.3) | 2.8 (1.3–5.0)** |
| Sex | ||||
| Male | 143 (50) | 55 (63) | 1.0 | 1.0 |
| Female | 143 (50) | 33 (38) | 1.7 (1.02–2.7) | 1.6 (0.93–2.6) |
| Nationality | ||||
| Non–Ugandan | 281 (98) | 84 (96) | 1.0 | 1.0 |
| Ugandan | 5 (2) | 4 (5) | 0.37 (0.098–1.4) | 0.6 (0.1–2.4) |
| Have chronic illnesses | ||||
| Yes | 73 (26%) | 30 (34) | 1.0 | 1.0 |
| No | 213 (75%) | 58 (66%) | 0.7 (0.4–1.2) | 0.4 (0.2–1.2) |
| Currently on any
long-term medication |
||||
| Yes | 44 (24) | 62 (71) | 1.0 | 1.0 |
| No | 142 (76) | 26 (30) | 0.4 (0.2–0.8) | 0.7 (0.4–1.3) |
| Ever had previous
reactions to vaccinations |
||||
| Yes | 17 (6) | 2 (2) | 1.0 | 1.0 |
| No | 251 (88) | 79 (90) | 0.3(0.09–1.7) | 0.6(0.1–2.6) |
| Not sure | 18 (6) | 7 (8) | 0.3 (0.09–1.7) | 0.5 (0.1–2.9) |
| Usually have reactions
to any medicine |
||||
| Yes | 37 (13) | 10 (11) | 1.0 | 1.0 |
| No | 249 (87) | 78 (89) | 0.9 (0.4–1.8) | 0.8 (0.3–1.7) |
| Illness at the time you
received the COVID-19 vaccine |
||||
| Yes | 40 (14) | 7 (8) | 1.0 | 1.0 |
| No | 246 (86) | 81 (92) | 1.6 (0.6-3.9) | 1.0 (0.4–3.1) |
Note: COR= Crude Odds Ratio, AOR= Adjusted Odds Ratio, * p<0.05, ** p<0.01, *** p<0.001
Discussion
In our study, 77% of the participants reported having experienced an adverse event after receiving either dose of the vaccine. The most commonly experienced adverse events were injection site events, headache and fever. Most adverse events had an onset within 3 days of receiving the vaccine and most events lasted between 1-3 days. In addition to that, those aged 20–29 years, 30-39 years and 40-49 years were more likely to develop adverse events than those aged ≥50 years. No serious adverse events were reported.
Most participants in our study revealed that they experienced an adverse event after either dose of the vaccine. Based on reports for most vaccines, an immune response is induced after AstraZeneca vaccination (21–23). These immune responses can lead to adverse events and these events occur in different persons differently (23,24). In a prospective single-cohort study in Ethiopia among health workers to assess for adverse events after receiving the Oxford-AstraZeneca vaccine, 68% of the participants reported an adverse event (25).
Despite a difference in the study designs, the results were similar. Similarly, in Togo, 72% of participants reported at least one adverse event after vaccination with AstraZeneca vaccine (ChAdOx1 nCoV-19 vaccine). However, in an online cross-sectional study assessing for self-reported adverse events in Bangladesh, 51% of the participants reported having experienced an adverse event after receiving a dose of the Oxford-AstraZeneca (Covishield) vaccine (26). This proportion might have been lower due to the nature of the study.
In an online interview, a participant may not be able to freely express their symptoms. On the contrary, in a more engaging mode of communication such as phone interviews used in this study, participants may be led on to remember or recall some of the adverse events experienced. Furthermore, in an online interview, the participants may respond hurriedly thereby missing out on some adverse events experienced.
We also compared proportions of individuals who experienced adverse events with other vaccines. An online cohort study in the United States analyzing with participant-reported adverse events after COVID-19 vaccination revealed that 65% reported an adverse effect after receiving Pfizer–BioNTech COVID-19 vaccine (BNT162b2) and 80% after Johnson % Johnson vaccine (27). A large-scale community-based study in the United Kingdom reported an even much lower rate of 33.7% (28). The variation may be due to the difference in study design and the heterogeneity of the populations. Some evidence has pointed towards ethnic differences and vulnerability to adverse events following vaccination (29).
We found that the most commonly reported adverse events included injection site events, fever, headaches, and general body weakness. This is because the vaccine instructs the body immune system to react in certain ways including increases in blood flow more at the injection site, so more immune cells can circulate, and it raises the body temperature. This is consistent with other studies that reported similar reported adverse events among the population (27,30–32). In most cases, adverse events are expected and people receiving the vaccine need to be sensitized. This helps to provide assurance and prepare them psychologically. Furthermore, health workers were also trained in managing adverse events as most are expected.
No serious adverse events were reported in our study. We believe this is due to short-lived, self-limiting symptoms that are mild or moderate in severity. Most adverse events were self-resolved with none requiring inpatient hospitalization, resulting in death, a permanent/persistent disability or a congenital anomaly. A study in Ethiopia on adverse events among health care workers who received the Oxford/AstraZeneca vaccine noted similar results to our study (25). Several studies have also reported no serious adverse events following vaccination with AstraZeneca (28,33).
In China, a meta-analysis of 12 different vaccines at phase 3 level of clinical trials revealed higher odds of serious adverse events following at least one dose of mRNA vaccines (AOR: 1.47; 95% CI: 0.65–3.3) compared to those who received at least one dose of non-replicating viral vector vaccine (AOR: 0.76; 95% CI: 0.62–0.93) and inactivated vaccines (AOR: 0.79, 95% CI: 0.62–1.00) (34). However, the same study revealed that no solid evidence indicated that COVID-19 vaccines directly caused serious adverse events.
In our study, individuals aged 20–29 years, 30-39 years and 40-49 years were more likely to develop adverse events than those aged ≥50 years. Furthermore, the odds increased with decreasing age. This may be due to higher reactogenicity among younger people than their older. Younger individuals tend to have a more active immune system which wanes with increasing age (35). Similarly, several studies have noted increasing odds of adverse events among younger individuals following administration of AstraZeneca COVID-19 vaccine and other COVID-19 vaccines (25,31,33,36).
A comparative study among 3 different vaccines noted similar results (37). However, older individuals might report less adverse events as they often dismiss them as symptoms of old age. This has been shown in a non-COVID-19 related study (38). This implies that adverse events among elder individuals should be well-probed. Furthermore, older individuals and their caretakers where necessary should be sensitized to always seek medical advice where adverse events present.
Study limitations
First, phone interviews were voluntarily responded to by individuals; thus, they might not have been objective. Second, the data collected might also have been subjected to recall bias as a few weeks had passed for some participants before they were subjected to the questionnaire. This was addressed through proper explanation of the study purpose and detailed probing without leading the participants onto what to say during the interview. We also called back participants to seek for clarity in case it was necessary. Use of a prospective study would alleviate this study limitation.
Conclusions
Most individuals experienced at least one adverse event after receiving either dose of the AstraZeneca COVID-19 vaccine. The most common adverse events were injection site events, headache and fever. No serious adverse events occurred. Younger age (<50 years) was significantly associated with developing an adverse event compared to those aged 50 years and above.
Recommendations
We recommend the use of the AstraZeneca COVID-19 vaccines in Uganda to help curb the spread of the COVID-19 infection based on its safety.
Conflict of Interest
The authors declare that they had no competing interests.
Acknowledgements
We thank the respondents for their participation in the study. We also thank the District Health Teams of Kampala, Wakiso, and Mukono districts for the administrative clearance provided before conducting this study. Furthermore, we convey our appreciation to health workers at the different vaccination sites for cooperating with us during the study.
References
- Coronavirus disease (COVID-19) [Internet]. [cited 2021 Oct 13]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020;1–4. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
- Larsen JR, Martin MR, Martin JD, Kuhn P, Hicks JB. Modeling the Onset of Symptoms of COVID-19. Front Public Heal. 2020 Aug 13;0:473.
- ALIMOHAMADI Y, SEPANDI M, TAGHDIR M, HOSAMIRUDSARI H. Determine the most common clinical symptoms in COVID-19 patients: a systematic review and meta-analysis. J Prev Med Hyg [Internet]. 2020 Oct 6 [cited 2021 Oct 13];61(3):E304. Available from: /pmc/articles/PMC7595075/
- Comas-Herrera A, Ashcroft EC, Lorenz-Dant K, Ashcroft EC. International examples of measures to prevent and manage COVID-19 outbreaks in residential care and nursing home settings. 2020 [cited 2021 Oct 13]; Available from:https://www.nejm.org/doi/full/10.1056/NEJMoa2008457
- Maqbool A, Khan NZ. Analyzing barriers for implementation of public health and social measures to prevent the transmission of COVID-19 disease using DEMATEL method. Diabetes Metab Syndr Clin Res Rev. 2020 Sep 1;14(5):887–92.
- Reeves DB, Bracis C, Swan DA, Moore M, Dimitrov D, Schiffer JT. Rapid vaccination and early reactive partial lockdown will minimize deaths from emerging 4 highly contagious SARS-CoV-2 variants 5. medRxiv [Internet]. 2021 Feb 3 [cited 2021 Apr 16];2021.02.02.21250985. Available from: https://doi.org/10.1101/2021.02.02.21250985
- Prüβ BM. Current State of the First COVID-19 Vaccines. Vaccines 2021, Vol 9, Page 30 [Internet]. 2021 Jan 8 [cited 2021 Nov 3];9(1):30. Available from: https://www.mdpi.com/2076-393X/9/1/30/htm
- COVID-19 vaccines [Internet]. [cited 2021 Apr 16]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Vol. 397, The Lancet. Elsevier B.V.; 2021. p. 1023–34.
- CDC. Understanding Adverse Events and Side Effects | Vaccine Safety | CDC [Internet]. [cited 2021 Nov 9]. Available from: https://www.cdc.gov/vaccinesafety/ensuringsafety/sideeffects/index.html
- World Health Organization (WHO). MODULE 3: Adverse events following immunization MODULE 3 Adverse events following immunization.
- What are Covid-19 vaccine adverse events and how are they managed? | News | Wellcome [Internet]. [cited 2021 Nov 9]. Available from: https://wellcome.org/news/covid-vaccine-adverse-events
- MODULE 3 – Vaccine reactions – WHO Vaccine Safety Basics [Internet]. [cited 2021 Nov 9]. Available from: https://vaccine-safety-training.org/vaccine-reactions.html
- WHO. Uganda receives 864,000 doses of COVID-19 vaccines | WHO | Regional Office for Africa [Internet]. 2021. [cited 2021 Nov 3]. Available from: https://www.afro.who.int/news/uganda-receives-864000-doses-covid-19-vaccines
- Unicef. Uganda receives first batch of AstraZeneca COVID-19 vaccines [Internet]. 2021 [cited 2022 Apr 21]. Available from: https://www.unicef.org/uganda/press-releases/uganda-receives-first-batch-astrazeneca-covid-19-vaccines
- WHO. COVID-19 Vaccines : Safety Surveillance Manual Module : Responding to adverse events.
- Side Effects of COVID-19 Vaccines [Internet]. [cited 2021 Apr 18]. Available from: https://www.who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines
- European Medicines Agency. COVID-19 Vaccine AstraZeneca: PRAC investigating cases of thromboembolic events – vaccine’s benefits currently still outweigh risks – Update | European Medicines Agency [Internet]. 2021 [cited 2021 Nov 9]. Available from: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-investigating-cases-thromboembolic-events-vaccines-benefits
- Daily Monitor. Covid vaccine safe, says government | Monitor [Internet]. 2021 [cited 2022 Apr 22]. Available from: https://www.monitor.co.ug/uganda/news/national/covid-vaccine-safe-says-government-3324892
- Science Brief: SARS-CoV-2 Infection-induced and Vaccine-induced Immunity | CDC [Internet]. [cited 2022 Mar 21]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html
- Immune response after COVID-19 vaccination | British Society for Immunology [Internet]. [cited 2022 Mar 21]. Available from: https://www.immunology.org/coronavirus/connect-coronavirus-public-engagement-resources/immune-response-after-covid-19
- Rose R, Neumann F, Grobe O, Lorentz T, Fickenscher H, Krumbholz A. Humoral immune response after different SARS-CoV-2 vaccination regimens. BMC Med [Internet]. 2022 Dec 1 [cited 2022 Mar 21];20(1):1–13. Available from: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-021-02231-x
- Side Effects of COVID-19 Vaccines [Internet]. [cited 2022 Mar 21]. Available from: https://www.who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines
- Tequare MH, Abraha HE, Adhana MT, Tekle TH, Belayneh EK, Gebresilassie KB, et al. Adverse events of Oxford/AstraZeneca’s COVID-19 vaccine among health care workers of Ayder Comprehensive Specialized Hospital, Tigray, Ethiopia. IJID Reg. 2021 Dec 1;1:124–9.
- Sultana A, Shahriar S, Tahsin MR, Mim SR, Fatema KR, Saha A, et al. A Retrospective Cross-Sectional Study Assessing Self-Reported Adverse Events following Immunization (AEFI) of the COVID-19 Vaccine in Bangladesh. Vaccines 2021, Vol 9, Page 1090 [Internet]. 2021 Sep 28 [cited 2022 Feb 8];9(10):1090. Available from: https://www.mdpi.com/2076-393X/9/10/1090/htm
- Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, et al. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw Open [Internet]. 2021 Dec 1 [cited 2022 Mar 21];4(12):e2140364–e2140364. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2787361
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis [Internet]. 2021 Jul 1 [cited 2022 Mar 21];21(7):939–49. Available from: http://www.thelancet.com/article/S1473309921002243/fulltext
- Baehr A, Peña JC, Hu DJ. Racial and Ethnic Disparities in Adverse Drug Events: A Systematic Review of the Literature. J racial Ethn Heal disparities [Internet]. 2015 Dec 1 [cited 2022 Mar 23];2(4):527–36. Available from: https://link.springer.com/article/10.1007/s40615-015-0101-3
- Jeon M, Kim J, Oh CE, Lee JY. Adverse Events Following Immunization Associated with Coronavirus Disease 2019 Vaccination Reported in the Mobile Vaccine Adverse Events Reporting System. J Korean Med Sci [Internet]. 2021 May 1 [cited 2022 Mar 21];36(17):1–8. Available from: https://pubmed.ncbi.nlm.nih.gov/33942578/
- Konu YR, Gbeasor-Komlanvi FA, Yerima M, Sadio AJ, Tchankoni MK, Zida-Compaore WIC, et al. Prevalence of severe adverse events among health professionals after receiving the first dose of the ChAdOx1 nCoV-19 coronavirus vaccine (Covishield) in Togo, March 2021. Arch Public Health [Internet]. 2021 Dec 1 [cited 2022 Mar 21];79(1). Available from: https://pubmed.ncbi.nlm.nih.gov/34819146/
- Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, et al. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J Clin Biochem [Internet]. 2021 Oct 1 [cited 2022 Mar 21];36(4):427. Available from: /pmc/articles/PMC7997788/
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet (London, England) [Internet]. 2021 Dec 19 [cited 2022 Mar 22];396(10267):1979–93. Available from: https://pubmed.ncbi.nlm.nih.gov/33220855/
- Fan Y, Chan KH, Hung IFN. Safety and efficacy of COVID-19 vaccines: A systematic review and meta-analysis of different vaccines at phase 3. Vaccines [Internet]. 2021 Sep 1 [cited 2022 Apr 22];9(9). Available from: /pmc/articles/PMC8473448/
- Lawton G. You’re only as young as your immune system. New Sci [Internet]. 2020 Mar 28 [cited 2022 Mar 23];245(3275):44. Available from: /pmc/articles/PMC7270427/
- Jose M, Rajmohan P, Thomas J, Krishna S, Antony B, U G U, et al. Active Symptom-Based Surveillance of Adverse Events Following Immunization among Individuals Vaccinated with ChAdOx1 nCoV-19 Coronavirus Vaccine in a Tertiary Hospital of Kerala. Curr Drug Saf [Internet]. 2022 Feb 9 [cited 2022 Mar 23];17. Available from: https://pubmed.ncbi.nlm.nih.gov/35135453/
- Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab Syndr Clin Res Rev. 2021 Sep 1;15(5):102207.
- Cahir C, Wallace E, Cummins A, Teljeur C, Byrne C, Bennett K, et al. Identifying Adverse Drug Events in Older Community-Dwelling Patients. Ann Fam Med [Internet]. 2019 Mar 1 [cited 2022 Mar 23];17(2):133–40. Available from: https://www.annfammed.org/content/17/2/133
![]()
![]()
![]()
![]()
![]()
![]()


Comments are closed.