
A Suspected Intussusception Outbreak among Children, Kampala District, March 2018.
Authors: Carol Nanziri1, Godfrey Nsereko1, Dan Kadobera1, Alex Riolexus Ario1; Affiliations: 1Uganda Public Health Fellowship Program, Kampala, Uganda
Summary
Intussusception is a common cause of small bowel obstruction in children under 2 years, characterized by colicky abdominal pain, vomiting, and bloody diarrhea. A social media report from a children’s clinic in Wakiso District reported a 3 fold increase in cases seen the previous year 2017. We conducted an investigation to verify the existence of an outbreak, determine the scope, cause and risk factors associated with the outbreak, and recommend measures to control this outbreak. A suspected case was defined as occurrence of surgery for intussusception in any child under 5 years of age at a Kampala District Hospital from January 2016 to March 2018. We reviewed surgical records for children less than 5 years of age
who had surgery for intussusception between January 2016 and March 2018 from 5 hospitals in Kampala District. We compiled a line list of demographic and surgical data for 130 intussusception cases. 103/130 (78%) cases were under 12 months with the median age of 6 months (range 0-60). The male to female ratio was 1.5:1. There was a total of 6 deaths with 4 occurring in other districts outside Kampala. There was no evidence of an outbreak in Kampala District and no specific pattern of intussusception cases seen throughout each year. We recommend establishment of routine surveillance to monitor safety of rotavirus vaccine.
Background
Intussusception is the invagination of one segment of the bowel into a distal segment. It can result in obstruction, vascular compromise, and necrosis of the intestine, and can lead to death if untreated. Approximately two-thirds of cases occur in children less than 1 year, with a peak incidence from 3 to 6 months of age. Diagnosis is made by air or liquid contrast enema, abdominal ultrasound, or during surgery or autopsy. In 1999, a first generation oral rotavirus vaccine, Rotashield, was with drawn from the US market because of a significantly increased risk of intussusception occurring after its administration (1)(2). However, preclinical safety trials for the current World Health Organization (WHO) prequalified rotavirus vaccines, Rotarix and RotaTeq, identified no vaccine attributable intussusception risk (3) (4). Recent studies from the US, Australia, Brazil, and Mexico, however, have shown 1- to 5-fold increased risk of intussusception following vaccination with Rotarix and RotaTeq (5) (6) (7) (8) (9) . Following a social media report on the 16th March 2018 of increased intussusception cases from a children’s clinic in Wakiso district, we conducted an investigation to verify the existence of an outbreak, determine the scope, cause and risk factors associated with the outbreak, and recommend measures to control this outbreak.
Methods
We defined a case as a surgical operation for intussusception on any child less than 5 years of age in a Kampala District hospital between January 2016 and March 2018. Kampala is a centrally located metropolitan District mostly bordered by Wakiso District in the South, North and West and only Mukono District in the East. This investigation focused on Kampala District because the clinicians in the Wakiso District Children’s clinic referred all suspected case patients to Hospitals in Kampala (i.e Nakasero and Mulago Hospitals). Wakiso district population is known to seek medical services in Kampala district due to its metropolitan location. We conducted a surgical records review in 5 Major referral hospitals including Mulago National Referral, Naguru, Case, Nsambya and Nakasero Hospitals. We compiled a line list with demographic, surgical and outcome data from case persons between January 2016 and March 2018. We did a descriptive analysis by person, place and time.
Findings
Time Distribution
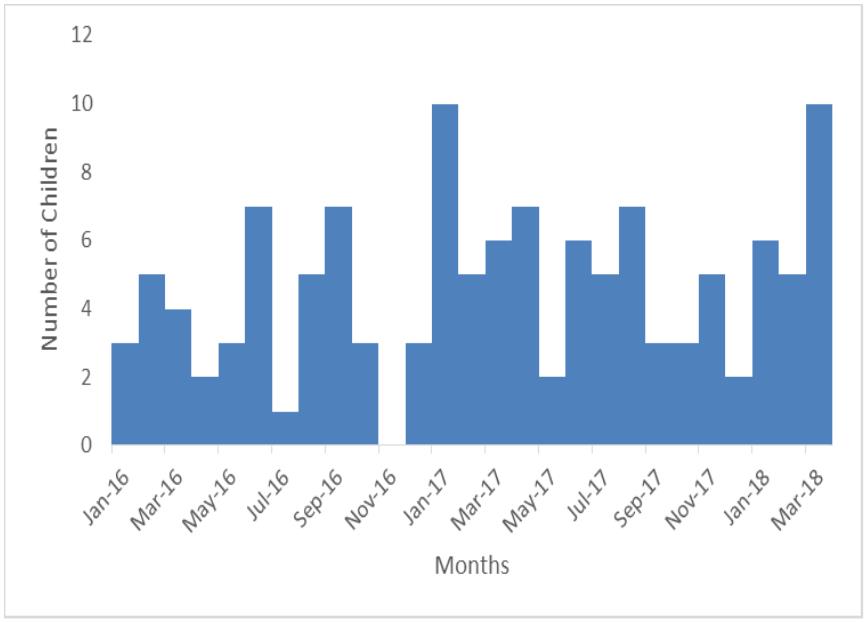
No of intussusception cases fluctuated throughout the year with no specific pattern
Person distribution
103/130 (78%) cases were under 12 months old with the median age of 6 months (range 0 – 60 months). The male to female ratio was 1.5: 1.
| Age in Months | Number of Cases | Percentage |
| 0 to 12 | 102 | 78% |
| 13 to 60 | 28 | 22% |
| Total | 130 | 100% |
Table 1: Distribution of Intussusception by age between January 2016 and March 2018
| Sex | Number of Cases | Percentage |
| Male | 77 | 59% |
| Female | 53 | 41% |
| Total | 130 | 100% |
Table 2: Distribution of Intussusception cases by Sex between January 2016 and March 2018
Discussion
The monthly rate of intussusception cases fluctuated throughout the year with no visible pattern. There were several peaks of 5 cases every after 2 months with the highest peak of 10 cases in January 2017 and March 2018. These findings differ from studies done in Ethiopia and Sub tropical China that showed seasonal variability with cases presenting during wetter months (10) (11).
This could be due to the short 2 year period of records studied compared to a longer 4 to 5 year period used in these studies. Other studies found no seasonal variability of intussusception among children less than a year old. This investigation did not establish the association of Rota Vaccine to intussusception.
The male to female ration in this investigation was 1.5:1. This reflects no clear difference in occurrence of intussusception by sex. A similar study carried out in Chennai city of India found a male to female ratio of 1.8:1 (12). 78% of the children with intussusception were under 1 year of age. These findings are similar to studies done in Ethiopia and Kenya which found over 60 percent of children with intussusception were younger than one year old (13) . This has been associated with more frequent lower respiratory tract infections as maternal antibodies wane after 5 months of age.
For this reason all vaccines are given before 12 months of age and mostly before 6 months of age. Although Rota Virus vaccine has been associated with an increased risk of intussusception in children under 4 months of age, this investigation was not able to verify its use among the children seen. Never the less, since it has been available for use in most of Kampala’s Private Hospitals for several years, its contribution to intussusception in this situation cannot be completely ignored.
Conclusion and recommendations
There was not enough evidence to establish presence of an intussusception outbreak in Kampala. We recommend increased public awareness for early diagnosis and treatment. Collection of rotavirus vaccine data among all cases of intussusception in the country will be useful to assess impact of rotavirus vaccine on intussusception.
References
- Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001 Feb 22;344(8):564–72.
- Peter G, Myers MG, National Vaccine Advisory Committee National Vaccine Program Office. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics. 2002 Dec;110 (6):e67.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006 Jan 5;354(1):23–33.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, vBreuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006 Jan 5;354(1):11–22.
- Buttery JP, Danch in MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011 Apr 5;29(16):3061–6.
- Patel MM, López-Collada VR, Bulhões MM, De Oliveira LH, Bautista Márquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011 Jun 16;364(24):2283–92.
- Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014 Feb 6;370(6):513–9.
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014 Feb 6;370(6):503–12.
- Tate JE, Mwenda JM, Armah G, Jani B, Omore R, Ademe A, et al. Evaluation of Intussusception after Monovalent Rotavirus Vaccination in Africa. N Engl J Med. 2018 19;378(16):1521–8.
- Gadisa A, Tadesse A, Hailemariam B. Patterns and seasonal variation of intussusception in children: A retrospective analysis of cases operated in a Tertiary Hospital in Ethiopia. Ethiopian medical journal. 2016 Jan 1;54.
- Guo W, Zhang S, Li J, Wang J. Association of Meteorological Factors with Pediatric Intussusception in Subtropical China: A 5 Year Analysis. PLOS ONE. 2014 Feb 28;9(2):e90521.
- Intussusception in children – UpToDate [Internet]. [cited 2018 Mar 26]. Available from: https://www.uptodate.com/contents/intussusception-in-children/print
- Gargano LM, Tate JE, Parashar UD, Omer SB, Cookson ST.
