Rapid assessment of knowledge and perceptions regarding ebola virus disease and infection prevention and control among health care workers, Uganda, November – December 2022
Authors: Mercy Wendy Wanyana1*, Alice Asio1, Rebecca Akunzirwe1, Patrick King1, Richard Migisha1, Benon Kwesiga1, Daniel Kadobeera1, Lilian Bulage1, Alex Riolexus Ario1 Institutional affiliations: 1Uganda Public Health Fellowship Program-Uganda National Institute of Public Health, Kampala, Uganda *Correspondence: Email: mwanyana@uniph.go.ug, Tel: +256779207928
Summary
Background: The absence of appropriate knowledge of Ebola Virus Disease (EVD) and infection prevention and control measures among healthcare workers can fuel EVD Healthcare-associated Infections (HAIs). We conducted a rapid assessment on knowledge and perceptions about EVD and IPC among healthcare workers working outside the Ebola Treatment Unit (ETU) in Kassanda and Kampala districts during the 2022 EVD outbreak in Uganda.
Methods: We conducted a cross-sectional study using quantitative methods in two outbreak districts of Kassanda and Kampala in Central Uganda. We interviewed 218 healthcare workers conveniently selected from 116 health facilities using a structured questionnaire in Central Uganda in November to December 2022. Variables considered included: knowledge about EVD (symptoms, first symptoms, transmission), the transmission of EVD Health-care Acquired Infections (HAIs), IPC concepts (handwashing, environmental cleaning and disinfection, decontamination of medical equipment and devices, waste segregation), and perceived barriers to implementing IPC measures. A score >50% of responses correct was considered good knowledge. Descriptive statistics including percentages were generated.
Results: Respondents were mainly females (76%) and worked in lower-level health facilities (clinics, health centre II, and health centre III) (64%). Half of the respondents (51%) correctly identified the initial symptoms of EVD. The highest proportion attributed EVD HAIs to non-use of PPE with 9% citing non-cleaning and disinfection of beds and environmental surfaces. The majority (63%) felt sufficiently informed to suspect EVD case. Most (79%) reported that they would isolate the case with 5% indicating that they would call the alert number. Less than half correctly responded to questions on hand hygiene (25%), environmental cleaning and disinfection (30%), decontamination and sterilisation (44%), and waste segregation (17%). Overall, forty-four per cent (44%) of respondents had good knowledge about EVD while 43% had good knowledge of IPC concepts and measures.
Conclusion: Healthcare workers’ knowledge of EVD and IPC concepts and measures was suboptimal. The findings were used to develop tailored training addressing knowledge gaps, especially in recognising EVD suspects, notifying alerts, hand hygiene, environmental cleaning and disinfection and waste segregation.
Background
The health and safety of healthcare workers is an emerging global health priority as health workers play a key role in maintaining resilient health systems(1). Healthcare workers are potentially exposed to numerous harmful agents arising from risks they are exposed to in their work setting(2). This includes highly infectious agents such as the Ebola virus.
Ebola virus often causes severe and lethal Ebola virus disease (EVD) infections for which there is no known treatment(3). In the absence of appropriate knowledge and implementation of Infection Prevention and Control (IPCs) measures, health workers get infected through contact with blood and body fluids from symptomatic EVD patients or bodies of deceased EVD patients (4).
Numerous healthcare worker EVD infections and deaths have been documented in previous EVD outbreaks on the African continent. In the first EVD outbreak in the Democratic Republic of Congo, 15 out of the 17 health workers at the hospital where the index case was managed got infected while 11 died(5). In the 1995 Kikwit EVD outbreak in the Democratic Republic of Congo, 25% of the cases were healthcare workers with an attack rate of 9%(6).
In the 2000 EVD outbreak in Uganda 31 out of the 425 EVD infections occurred among healthcare workers(7). In the 2007/2008 Bundibugyo Ebola virus outbreak recorded 14 out of 42 confirmed infections were healthcare workers(8). In the 2013-2016 West Africa EVD outbreak, 2-38 % of cases in the various outbreak locations were healthcare workers(4). In the EVD outbreak in North Kivu and Ituri in 2018–2020, 160 health worker infections were recorded among the 3481 cases(9). Health workers have a 21 to 32 times higher risk of getting EVD than the general population(10).
Health worker EVD infections are often linked to a lack of clinical recognition of EVD and incorrect implementation of appropriate IPC measures(4). Health worker exposures often occur when health workers are exposed while providing general medical and nursing care to unrecognised EVD patients(4,11). Only 11% of EVD health worker infections are associated with exposure in the Ebola Treatment units further showing that most infections occur before cases are recognised(12). Lack of recognition of Ebola and incorrect implementation of IPC measures result from poor knowledge and misconceptions of the disease and how to protect themselves(13).
Uganda registered growing numbers of health workers getting infected and Healthcare-Associated Infections (HAIs) during the 2022 Sudan Ebola virus outbreak. By November 1st 2022, there were 18 health-care worker infections. This questioned the existing knowledge of EVD among healthcare workers and how to protect themselves outside the Ebola treatment unit. We conducted a rapid assessment to assess knowledge and perceptions about EVD and IPC among healthcare workers working outside the Ebola Treatment Unit (ETU) in Kassanda and Kampala districts to inform control and prevention measures.
Methods
We conducted a cross-sectional study using quantitative methods during an on-going outbreak in two affected districts of Kassanda and Kampala in central Uganda from November to December 2022. By November 2022, there were 46 confirmed EVD cases in Kassanda and 18 confirmed EVD cases in Kampala.
The rapid assessment was conducted in 116 health facilities with no Ebola treatment Unit in two outbreak districts (Kampala and Kassanda).
We estimated the sample size using the Kish .L formula(14) with the following assumptions: proportion of health workers with knowledge on physical contact with body fluids from an infected person would transmit the disease 19.7%(15), 95% level of confidence, and a design effect of 2. After inflation of 10% non-response rate, the calculated sample size was 534 to be interviewed. However, only 218 health workers were at the facilities by the time of the assessment.
Due to the low staffing levels in Kassanda District, we selected all health facilities. In Kampala we purposively sampled 87 facilities that were targeted in the Infection Prevention and Control strengthening project supported by the Infectious Disease Institute. This project aimed at strengthening implementation of IPC measures in Kampala. At each healthy facility, health workers were conveniently selected based on availability and consent at the time of the interview. No more than 10 health workers were selected from each health facility
We used a standardized questionnaire developed in the Democratic Republic of Congo (DRC) by the US CDC Centre of Disease Control and the World Health Organisation during 2018–2020 North Kivu and Ituri EVD outbreak. It was adapted to the Ugandan setting and approved by the National Infection Prevention Sub Pillar, Ministry of Health.
The questionnaire was designed in Kobocollect software. The questionnaire collected data on knowledge about EVD (symptoms, first symptoms, transmission), transmission of EVD Health-care Acquired Infections, IPC concepts (handwashing, environmental cleaning and disinfection, decontamination of medical equipment and devices, waste segregation), and perceived barriers to implementing IPC measures.
We downloaded data from KoboCollect in an Excel file and imported to STATA version 14 software for analysis. Descriptive statistics including means and percentages were used to describe the knowledge and perceptions about EVD and IPC among healthcare workers. We generated two composite variables on knowledge about EVD and knowledge about IPC concepts and measures based on a score of responses. A score of >50% correct responses was considered good knowledge while a score of ≤50 was considered poor knowledge.
This rapid assessment was in response to a public health emergency under the Ministry of Health Outbreak Analytics Cell and was therefore determined to be non-research. The Ministry of Health Uganda gave a directive to assess knowledge perceptions on EVD and IPC in order to inform on-going interventions. The office of the Center for Global Health, US Center for Disease Control and Prevention determined that this activity was not human subject research and that its primary intent was for public health practice or disease control.
Permission to conduct the assessment was also sought from the administrators of the selected health facilities. We sought verbal informed consent and assent from the respondents in an effort to prevent spread of EVD. They were all informed that their participation was voluntary and their refusal would not attract any negative consequences. Data which was collected did not contain individual personal identifiers as a way of ensuring confidentiality.
Results
Characteristics of health care workers during a study to assess knowledge and perceptions regarding ebola virus disease and infection prevention and control, Uganda, November–December 2022
A total of 218 health workers participated in the rapid assessment. Most of the respondents were females (62%). Majority (64%) of respondents worked in lower level health facilities including clinic (11%), health centre II (15%), and health centre III (39%) in comparison to higher level facilities that is general hospitals (4%), national referral hospital (2%), and regional referral hospital (1%).
Forty-four percent (44%) of respondents worked in government owned health facilities, while the rest worked in private not for profit health facilities (26%), and private for profit health facilities (30%). Most respondents worked in health facilities located in urban areas (76%) in comparison to health facilities located in rural areas (24%).
Knowledge and perceptions of ebola virus disease
Knowledge of symptoms of ebola virus disease
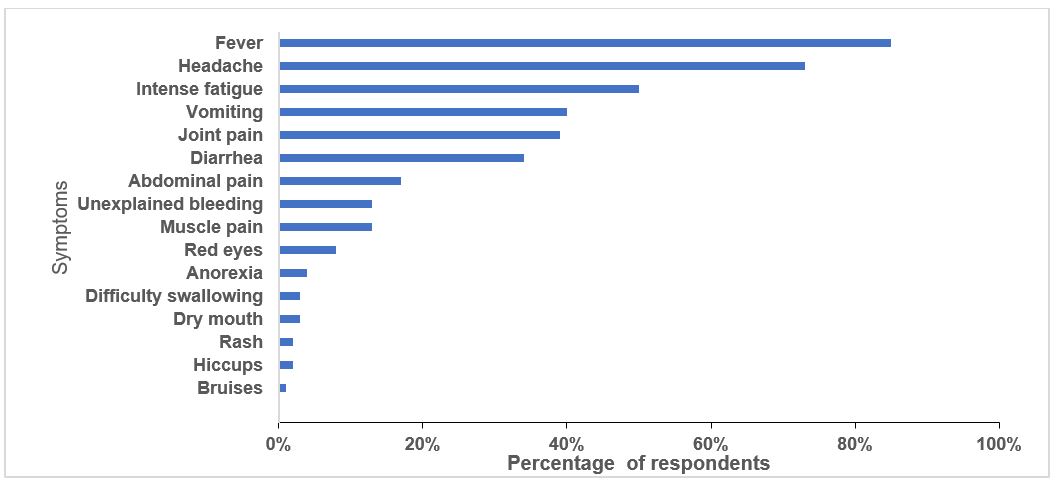
Half (56%) of the respondents rated their level knowledge as good, 32% as medium and 12% as low. Respondents listed all EVD symptoms, bruises (1%), red eyes (10%) and anorexia (14%) were the least listed symptoms in comparison with dry mouth (30%), muscle pain (40%), hiccups (40%), spontaneous abortion (40%), joint pain (45%), intense fatigue (63%), difficulty in swallowing (70%), unexplained bleeding (76%), fever (80%), rash (80%), diarrhoea (80%), headache (83%) and vomiting (85%). Half (51%) respondents correctly identified the initial symptoms of ebola.
Respondents also listed diarrhoea, vomiting, unexplained bleeding or spontaneous abortions (Figure 1) which are not the initial symptoms of ebola. A quarter of the respondents acknowledged that it was difficult to differentiate EVD from other common illnesses in the early stages. Forty-two (42%) reported difficulties in recognising ebola symptoms among children.
Inability to communicate EVD symptoms by children was the most cited (56%) reason for difficulties in recognising ebola symptoms among children other reasons included symptoms being less visible in children (30%), children being unlikely to be known contacts (14%)
Knowledge on transmission of ebola virus disease
Respondents listed the following ways through which ebola virus disease was transmitted: handling bushmeat (34%), physical contact with a person with ebola or their body fluids (93%), contact with dead bush animals (39.5%), contact with sharp objects or environment contaminated with body fluids of EVD patient (74%), sexual intercourse (55%), touching the bodies of people who have died of ebola (63%), transmitted because of the people who are paid to continue the spread of the disease (2%) and transmitted by response workers (3%).
Ebola virus disease health-care associated infections
Health worker behaviours attributed to EVD HAIs included; no use of Personal Protective Equipment (PPE) (43%) compared to non-sterilization of materials (20%), inability to recognize EVD patients (17%), non-cleaning and disinfecting beds and environmental surfaces (9%), bed sharing (4%), sharing food (1%), sharing a room (5%) and sharing or using needles (1%). Most respondents (78%) felt that children had an increased risk for getting EVD HAIs
Perceptions about management approach to handling a suspect
Majority (63%) of respondents felt sufficiently informed to suspect an EVD case. If they received one on the ward respondents reported that they would do the following: isolate the patient (79%), refer the patient to another health facility (18%), taking charge of the patient (11%), call the alert number (5%), and request the patient to go to an ETU (21%).
Overall level of knowledge about ebola virus disease
Overall, we found that 44% had good knowledge about EVD. The highest proportion of respondents had good knowledge in community transmission of EVD infections (58%) in comparison to EVD symptoms (54%) and transmission of EVD HAIs (19%) (Table 1)
Table 1: Level of knowledge regarding ebola virus disease among health care workers, Uganda, November–December 2022
| Level of knowledge about EVD | n | % |
| Overall Knowledge on EVD (Total score =29)
≤50% >50% |
122 96 |
56 44 |
| EVD symptoms (Total score =17)
≤ 50% > 50% |
100 118 |
46 54 |
| Transmission of community EVD infections (Total score= 5)
≤ 50% > 50% |
92 126 |
42 58 |
| Transmission of EVD HAI (Total score=9) | ||
| ≤ 50%
> 50% |
176
42 |
81
19 |
Knowledge on infection prevention and control measures and concepts among health care workers, Uganda, November–December
Less than half of respondents had knowledge in the various IPC concepts and measures. (Table 2). A quarter (25%) of respondents correctly listed all the times health workers should wash their hands (after providing patient care, before providing patient care, after touching a patient’s things/surfaces surroundings, after coming into contact with body fluids and before a patient procedure).
Thirty respondents correctly identified how to clean a body fluid spill on the floor by wiping organic material with towel/cloth; cleaning surface with soap and water; disinfecting with 0.5 chlorine for 10 min; rinse with water. Thirty-four (34%) of respondents cited using washing with 0.5% chlorine or soap without cleaning. Respondents incorrectly said they sterilized their medical equipment using chlorine (86%), water and soap (54%), boiling water (33%), simple water (16%) and firewood (11%).
Table 2: Knowledge on infection prevention and control measures and concepts among health care workers, Uganda, November–December
| IPC measures and concepts(N=218) | n | % |
| Hand washing | ||
| Please list all the times when a healthcare worker should wash their hands?
Correct response Incorrect response |
54 164 |
25 75 |
| Environmental Cleaning and disinfection | ||
| Can you explain how to clean a body fluid spill on the floor?
Correct Incorrect |
66 152 |
30 70 |
| Decontamination and sterilization of medical equipment and devices | ||
| How do you sterilize your medical instruments/equipment?
Correct Incorrect |
96 122 |
44 66 |
| Waste segregation | ||
| list all the groups you segregate your waste
Correct Incorrect |
181 37 |
83 17 |
Overall level of knowledge about infection prevention and control concepts and measures
Overall, we found that less than half (43%) had good knowledge about IPC measures and concepts. The highest proportion respondents had good knowledge in waste segregation (75%) in comparison to decontamination and sterilization of medical equipment and devices (44%), hand washing (46%) and environmental cleaning and disinfection (30%). (Table 4)
Table 4: Level of knowledge about infection prevention and control measures and concepts among health care workers, Uganda, November-December
| Level of knowledge about Infection Prevention and Control measures and concepts | n | % |
| Overall knowledge on infection prevention and control (Total score =13)
≤ 50% > 50% |
124 94 |
57 43 |
| Hand washing (Total score =6)
≤ 50% > 50% |
118 100 |
54 46 |
| Environmental cleaning and disinfection (Total score= 1)
≤ 50% > 50% |
152 66 |
70 30 |
| Decontamination and sterilization of medical equipment and devices (Total score=1) | ||
| ≤ 50%
> 50% |
122
96 |
66
44 |
| Waste segregation (Total score= 5)
≤ 50% > 50% |
55 163 |
25 75 |
Perceived barriers to implementing appropriate infection prevention and control measures
Barriers to implementing IPC measures included: lack of regular training (53%), insufficient IPC materials (46%), lack of regular supervision (24%), patients don’t like IPC practices or feel threatened by the community or patients for applying IPC measures (20%), not understanding the different IPC protocols (12%), not enough IPC staff (12%), no financial reward for extra work (10%), afraid to be infected with Ebola (8%), and lack utilities (electricity, water) (6%).
Discussion
Our findings indicate gaps in the level of knowledge about EVD including inability to identify initial EVD symptoms, transmission of EVD health care associated infections, and ability to manage suspected EVD cases. Furthermore, respondents indicated misconceptions in key IPC concepts with only a few respondents able to correctly respond. There were several perceived barriers to implementing including lack of regular training, insufficient IPC materials, lack of regular supervision, and fear of negative attitude from patients or community.
Similar to previous studies conducted in Uganda Nigeria and Ethiopia our study indicated low levels of knowledge of EVD(15–17). Although healthcare workers were knowledgeable in EVD symptoms, most could not correctly cite the initial presenting symptoms. These may lead to delayed recognition and misclassification of cases. Vomiting, diarrhoea, and bleeding were commonly cited yet these typically occur 4-5 days after the initial onset of symptoms(18). This may contribute to delayed isolation
increasing the likelihood of transmission. Only 5% reported that they would call the alert number. This could cause delays in notification and isolation of cases. Most health facilities at lower levels(Clinics and Health Centre II) in Uganda do not have isolation capacities(15). In such settings failure to recognise and report suspected EVD cases may fuel EVD HAIs.
We noted knowledge gaps on how Healthcare Associated EVD Infections occurred despite being knowledgeable in community transmission of EVD. Most health-care workers attributed EVD HAIs to non-use of PPE. Although this is critical it may not be enough to prevent these infections. Other IPC measures that most respondents did not cite are essential. Previous studies have indicated that the EVD virus can survive on environmental surfaces for 7-10 days highlighting the need for other behaviours such as cleaning disinfecting beds and environmental surfaces(19,20).
We noted knowledge gaps on IPC measures and misconceptions about key IPC concepts. Failure to identify all the critical times health care workers should wash their hands could lead to poor hand washing practices reducing the effectiveness of handwashing in protecting healthcare workers(21). Similarly failure to clean surfaces prior to disinfection may reduce the overall effectiveness of disinfectants used(22). We noted misconceptions about the differences between cleaning, disinfection and sterilisation. This may hinder appropriate application of these while implementing IPC measures(23).
Our findings highlighted several perceived barriers to implementing IPC measures. Like in other low resource settings, respondents highlighted insufficient IPC materials especially in emergency settings(24). Similar to a previous study conducted in Uganda, we found that patient perceptions about IPC measures especially PPE hindered healthcare workers from implementing IPC practices(25).
Limitations
Our findings should be interpreted with the following limitations. We were unable to achieve the desired sample size due to the low staffing levels in the health facilities and the fact was conducted at the peak of the outbreak. This may reduce the generalisability of our findings. However, the findings were targeted in inform interventions in the response area.
Conclusion
In conclusion, health care workers had inadequate knowledge on EVD and IPC measures. There is a need for tailored trainings to address knowledge gaps especially in recognising EVD suspects, notifying alerts, hand hygiene, environmental cleaning and disinfection and waste segregation.
Conflict of interest
The authors declare that they had no conflict of interest.
Acknowledgments
We thank the Ministry of Health, Public, and the United Nations Children’s Fund Integrated Outbreak Analytics Cell for supporting this assessment. We appreciate the technical support provided by the Ministry of Health Infection Prevention and Control Pillar. Finally, we thank the US-CDC for supporting the activities of the Uganda Public Health Fellowship Program.
Copyright and licensing
All materials in the Uganda Public Health Bulletin is in the public domain and may be used and reprinted without permission; citation as to source; however, is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form
References
- McDiarmid M. Advocating for the health worker. Ann Glob Heal. 2019;85(1).
- Mossburg S, Agore A, Nkimbeng M, Commodore-Mensah Y. Occupational Hazards among Healthcare Workers in Africa: A Systematic Review. Ann Glob Heal. 2019 Jun;85(1).
- Jacob ST, Crozier I, Fischer WA 2nd, Hewlett A, Kraft CS, Vega M-A de La, et al. Ebola virus disease. Nat Rev Dis Prim. 2020 Feb;6(1):13.
- Selvaraj SA, Lee KE, Harrell M, Ivanov I, Allegranzi B. Infection Rates and Risk Factors for Infection Among Health Workers During Ebola and Marburg Virus Outbreaks: A Systematic Review. J Infect Dis [Internet]. 2018 Nov 22;218(suppl_5):S679–89. Available from: https://doi.org/10.1093/infdis/jiy435
- WHO (World Health Organisation). Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56(2):271–93.
- Tomori O, Bertolli J, Rollin PE, Fleerackers Y, Guimard Y, De Roo A, et al. Serologic Survey among Hospital and Health Center Workers during the Ebola Hemorrhagic Fever Outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis [Internet]. 1999 Feb 1;179(Supplement_1):S98–101. Available from: https://doi.org/10.1086/514307
- CDC (US Centre for Disease Control). Outbreak of Ebola hemorrhagic fever Uganda, August 2000-January 2001. MMWR Morb Mortal Wkly Rep. 2001 Feb;50(5):73–7.
- Wamala JF, Lukwago L, Malimbo M, Nguku P, Yoti Z, Musenero M, et al. Ebola hemorrhagic fever associated with novel virus strain, Uganda, 2007-2008. Emerg Infect Dis. 2010 Jul;16(7):1087–92.
- Baller A, Padoveze MC, Mirindi P, Hazim CE, Lotemo J, Pfaffmann J, et al. Ebola virus disease nosocomial infections in the Democratic Republic of the Congo: a descriptive study of cases during the 2018–2020 outbreak. Int J Infect Dis [Internet]. 2022 Feb 1;115:126–33. Available from: https://doi.org/10.1016/j.ijid.2021.11.039
- Organization WH. Health worker Ebola infections in Guinea, Liberia and Sierra Leone: a preliminary report 21 May 2015. World Health Organization; 2015.
- Forrester JD, Hunter JC, Pillai SK, Arwady MA, Ayscue P, Matanock A, et al. Cluster of Ebola cases among Liberian and U.S. health care workers in an Ebola treatment unit and adjacent hospital — Liberia, 2014. MMWR Morb Mortal Wkly Rep. 2014 Oct;63(41):925–9.
- Olu O, Kargbo B, Kamara S, Wurie AH, Amone J, Ganda L, et al. Epidemiology of Ebola virus disease transmission among health care workers in Sierra Leone, May to December 2014: a retrospective descriptive study. BMC Infect Dis. 2015 Oct;15:416.
- Raven J, Wurie H, Witter S. Health workers’ experiences of coping with the Ebola epidemic in Sierra Leone’s health system: a qualitative study. BMC Health Serv Res [Internet]. 2018;18(1):251. Available from: https://doi.org/10.1186/s12913-018-3072-3
- Kish L. Survey sampling: John Willey and Sons. Inc NY. 1965;
- Kibuule M, Sekimpi D, Agaba A, Halage AA, Jonga M, Manirakiza L, et al. Preparedness of health care systems for Ebola outbreak response in Kasese and Rubirizi districts, Western Uganda. BMC Public Health [Internet]. 2021;21(1):236. Available from: https://doi.org/10.1186/s12889-021-10273-2
- Olowookere SA, Abioye-Kuteyi EA, Adepoju OK, Esan OT, Adeolu TM, Adeoye TK, et al. Knowledge, Attitude, and Practice of Health Workers in a Tertiary Hospital in Ile-Ife, Nigeria, towards Ebola Viral Disease. J Trop Med. 2015;2015:431317.
- Abebe TB, Bhagavathula AS, Tefera YG, Ahmad A, Khan MU, Belachew SA, et al. Healthcare Professionals’ Awareness, Knowledge, Attitudes, Perceptions and Beliefs about Ebola at Gondar University Hospital, Northwest Ethiopia: A Cross-sectional Study. J Public Health Africa. 2016 Dec;7(2):570.
- Velásquez GE, Aibana O, Ling EJ, Diakite I, Mooring EQ, Murray MB. Time From Infection to Disease and Infectiousness for Ebola Virus Disease, a Systematic Review. Clin Infect Dis [Internet]. 2015 Oct 1;61(7):1135–40. Available from: https://doi.org/10.1093/cid/civ531
- Westhoff Smith D, Hill-Batorski L, N’jai A, Eisfeld AJ, Neumann G, Halfmann P, et al. Ebola Virus Stability Under Hospital and Environmental Conditions. J Infect Dis. 2016 Oct;214(suppl 3):S142–4.
- Cook BWM, Cutts TA, Nikiforuk AM, Poliquin PG, Court DA, Strong JE, et al. Evaluating environmental persistence and disinfection of the Ebola virus Makona variant. Viruses. 2015 Apr;7(4):1975–86.
- Organization WH. Guideline on hand hygiene in health care in the context of filovirus disease outbreak response : rapid advice guideline, November 2014. World Health Organization; 2014.
- (CDC) C for DC and P. Factors Affecting the Efficacy of Disinfection and Sterilization. 2008.
- Rutala WA, Weber DJ. Disinfection and Sterilization in Health Care Facilities: What Clinicians Need to Know. Clin Infect Dis [Internet]. 2004 Sep 1;39(5):702–9. Available from: https://doi.org/10.1086/423182
- Lowe H, Woodd S, Lange IL, Janjanin S, Barnet J, Graham W. Challenges and opportunities for infection prevention and control in hospitals in conflict-affected settings: a qualitative study. Confl Health [Internet]. 2021;15(1):94. Available from: https://doi.org/10.1186/s13031-021-00428-8
- Raabe VN, Mutyaba I, Roddy P, Lutwama JJ, Geissler W, Borchert M. Infection control during filoviral hemorrhagic fever outbreaks: preferences of community members and health workers in Masindi, Uganda. Trans R Soc Trop Med Hyg. 2010 Jan;104(1):48–50.

