Outbreak of Malaria associated with Increased Commercial Activities around Swamps and Amplified Vector Density, Oyam District, Uganda, January-June 2019
Authors: Katusiime Maureen1, Gerald Rukundo1, Steven Ndugwa Kabwama 1, Alex Riolexus Ario 1: Affiliations: 1Uganda Public Health Fellowship Program, Ministry of Health, Kampala, Uganda
Summary
In June 2019, Oyam District revealed an upsurge of malaria cases beyond the action threshold. We investigated to determine the scope of the out- break, identify exposures associated with transmission and recommend evidence-based control measures. We defined a confirmed case as a positive malaria rapid diagnostic test (mRDT) or malaria microscopy from 1 January-30 June 2019 in a resident or visitor of Acaba Sub-county, Oyam District. We identified cases by reviewing records at health facilities in Acaba Sub-county. We interviewed case-patients and asymptomatic age- and parish-matched controls to determine exposures associated with illness. We conducted entomological and environmental assessments in the most affected villages within the Acaba Sub-county. We found 9,235 confirmed case-persons (AR=29%) and 1 death. Females (AR=38%) were affected more than males (AR=20%) (p<0.001). Children <18 years were more affected (AR=37%) than adults >18 years (AR=27%) (p<0.001).
Among 83 case-patients and 83 controls, 66 (80%) case-patients and 33 (40%) controls engaged in commercial ventures <500m from a swamp (ORMH=12, 95%CI 3.7-39); 18 (22%) case-patients and four (5%) controls lived <500m from rice irrigation sites (ORMH=8.0, 95%CI 1.8-35); and 23 (28%) case-patients and four (5%) controls had water pools <100m from household for 3-5 days after rainfall (ORMH=7.3, 95%CI 2.2-25). Seventy (84%) controls and 60 (72%) case-patients used bed nets (ORMH=0.5, 95% CI 0.0-0.4); 40 (48%) controls and 15 (18%) case-patients wore long- sleeved clothes during evening hours (ORMH=0.12, 95%CI 0.0-0.4). Indoor resting vector density was 19 mosquitoes/household/night. All seven (100%) breeding sites observed <500m from households had Anopheles larvae; sand pits (25/250mls) and brick pits (16/200mls) had the highest average larvae concentrations. This outbreak was facilitated by breeding sites near homes and commercial ventures, compounded by poor use of individual preventive measures. We recommended removal of potential breeding sites, mass case management of malaria, and increasing comunity awareness on use of insecticide-treated bed nets and protective clothing
Background
Malaria remains a major public health problem with Uganda ranking 5th among the highest malaria-burden countries globally. Uganda also has some of the highest transmission rates in the world (1) and sixth highest number of deaths from malaria in Africa (2). Malaria accounts for 30-50% of outpatient visits at health facilities, 15-20% of all hospital admissions, and up to 20% of all hospital deaths of which 27.2% occur among children under five years of age in Uganda. In June 2019, routine analysis of surveillance data at Ministry of Health revealed an upsurge of malaria cases beyond the action threshold in the entire Oyam District with Acaba Sub County was the most affected. We investigated to determine the scope of outbreak, identify exposures for transmission, and recommend evidence-based control measures.
Methods
We defined a confirmed case as a positive malaria result using mRDT or microscopy from 1 January 2019 to 30th June 2019 in a resident or visitor of Acaba Sub-county in Oyam District, Uganda. We reviewed medical records in the out- patient registers to identify cases. We conducted descriptive epidemiology to describe the time, place, and person characteristics of the case persons. We conducted environmental and entomological assessments in the most affected villages to identify potential breeding sites and vector density respectively. We conducted hypothesis generating interviews in 20 households and a case control study to identify exposures of transmission among 83 cases and 83 controls, matched by age and parish.
Results
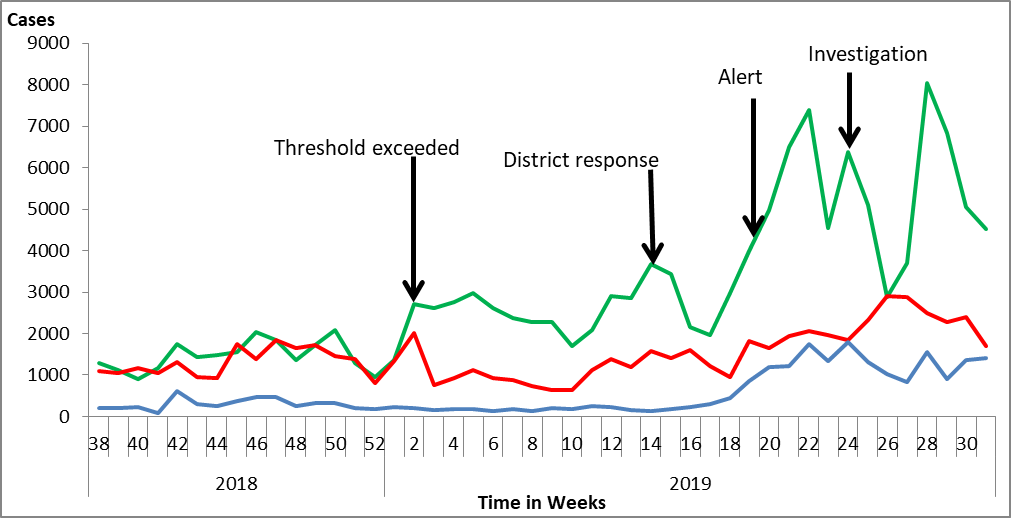
We found 9,235 confirmed case-persons (AR=29%) with median age 20 years (IQR: 6-21 years) and mean age 26.4 (SD±18.1). Obangangeo Parish was the most affected (AR=47%) followed by Abanya (AR=37%) and Dogapio (AR=36%). Females (AR=38%) were more affected than males (AR=20%). Children <18 years were more affected (AR=37%) than adults >18 years (AR=27%) The epidemic curve showed an increase in malaria cases from April, peaking in May and June. The rains were intermittent from February and increased for subsequent months (Figure 2).

Entomological assessment found Anopheles gambiae sensu lato and Anopheles funestus as the most common vectors; average indoor rest- ing density was 19 mosquitoes per house per night.
Hypothesis generating interviews found 95% of households had kitch- ens detached from main house, 95% did not use mosquito repellant, 68% had human activity within 100m from swamp and 63% entered bed after 9pm. We hypothesized that having human activity within 500m from swamp was associated with malaria infection.
Case control findings
Sixty seven percent (66/99) of case-patients engaged in commercial ventures like yam cultivation within 500m from swamp compared to 33% (33/99) of controls (ORMH= 12, 95%CI 3.7-39) two weeks before symptom onset; 82% (18/22) of case-patients lived within 500m from rice irrigation activity compared to 18% (4/22) of controls (ORMH=8, 95%CI 1.8-35 ) two weeks before symptom onset and 85% (23/27) of case patients had water pools around households for 3-5 days after rain- fall compared to 15% (4/27) of controls (ORMH=7.3, 95%CI 2.2-25). Furthermore, 46 %( 60/130) of case-patients used mosquito nets compared to 54% (70/130) of controls (ORMH=0.5, 95%CI 0.0-0.4) two weeks be- fore symptom onset; 27% (15/55) of case-patients wore long-sleeved clothes during evening hours compared to 40/55 (73%) of controls (ORMH=0.12, 95%CI 0.0-0.4) two weeks before symptom onset.
Discussion
Our investigation revealed an overall attack rate in Acaba sub county was 329 cases per 1000 population which was much higher than the incidence of malaria in Uganda of 191/1000 population (4) and AR of 12.1/1000 in Northern Ethiopia (5). The high AR in our study might be attributed to area difference in the burden of malaria and duration of the illness. Oyam District is a high transmission area for malaria and the duration of illness was long compared to the Ethiopian study.
People whose houses were within 500m to 1 km from breeding sites (swamp, rice irrigation and yam growing) were more likely to have ma- laria infection than those beyond 1km. This finding is consistent with those in Ethiopia (6). A possible explanation is the fact that most vec- tors travel shorter distances from breeding sites. A Gambian study found that the number of mosquitoes caught in houses declined steeply with distance to nearest breeding habitat (7). Secondly, possibly human activity within 500m from existing swamps increased the number of breeding sites which could have created desirable conditions for the multiplication of the vectors that bit and infected people. These sites hold fresh stagnant water that provides active breeding sites for malaria vectors (1).
In addition, water logging around household for 3-5 days after rainfall and having empty containers around household were independently associated with malaria. These risk factors increase the number of breeding sites for the mosquitoes around the home similar to findings of a malaria outbreak investigation conducted in Ethiopia (5). Sleeping under treated nets and wearing long clothes to cover arms and legs provided significant protection against malaria. Personal protection to prevent vector contact is effective, affordable, and simple to apply. Hence the continued need to integrate such strategies into routine awareness and behavior change communication messages to fight against malaria.
Conclusion and recommendations
This outbreak was associated with human activity around swamps which increased breeding sites for vectors coupled with increased mosquito density. We recommended vector surveillance and larval source management, Mass Action against Malaria including increasing awareness of community on malaria prevention measures through behavioral change communication (BCC), interpersonal communication (IPC) and Information Education and Communication (IEC).
References
- World Health Organization.(2018) WORLD MALARIA RE- PORT 2018. 2018.
- MoH, (2014) THE UGANDA MALARIA REDUCTION STRA- TEGIC PLAN 2014-2020
- Mawejje H.D, Wilding C.S, Rippon E.J, Hughes A, Weetman. D, Donnelly M.J (2013) Insecticide resistance monitoring of fieldcollected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Medical and Veterinary Entomology. 2013 Sep;27(3):276-83.
- MoH Surveillance Monitoring and Evaluation Unit (2019) National Malaria Annual Report 2017-2018, Kampala, Uganda. http://health.go.ug/Publications
- Tesfahunegn. A., Gebretsadik. B and Gebregziabher. E (2019) Risk factors associated with malaria outbreak in Laelay Adyabo district northern Ethiopia, 2017: Case- Control Study Design. BMC Public Health (2019) 19:484 https://doi.org/10.1186/s12889-019-6798-x
- Thomas CJ, Cross DE, Bøgh C (2013) Landscape Movements of Anopheles gambiae Malaria Vector Mosquitoes in Ru- ral Gambia. PLoS ONE 8(7): e68679. https://doi.org/10.1371/journal.pone.0068679
- Tukei BB, Beke A, Lamadrid-Figueroa H (2017) Assessing the effect of indoor residual spraying (IRS) on malaria mor- bidity in Northern Uganda: a before and after study. Ma- laria Journal volume 16, Article number: 4 (2017).


Comments are closed.