Gastrointestinal anthrax outbreak investigation, Ibanda District, Southwestern Uganda, August 2022
Authors: Patrick King1*, Brenda Nakafeero Simbwa1, Peter Chris Kawungezi1, Job Morukileng1, Mercy Wendy Wanyana1, Muhumuza Nicholas2, Munyaneza Godfrey2, Babirye Priscilla2, Asiimwe Grace Karim2, Winfred Nakaweesi2, Richard Migisha1, Daniel Kadobera1, Benon Kwesiga1, Lilian Bulage1 Institutional affiliations: 1Uganda Public Health Fellowship Program-Uganda National Institute of Public Health, Kampala, Uganda, 2Frontline Field Epidemiology Training Programme-Uganda National Institute of Public Health *Correspondence: Email: kingp@uniph.go.ug, Tel: +256775432193
Summary
Background: Anthrax outbreaks associated with exposures to meat from cows that die suddenly are increasingly common in Uganda, with three outbreaks reported during January-July 2022. In Uganda, animals that die suddenly are supposed be notified to authorities and disposed off safely. On August 23, 2022, the MoH was alerted about a human community death due to suspected anthrax in Ibanda District. In response, we investigated to determine the scope of the outbreak and possible exposures, and recommend suitable measures to control the outbreak.
Methods: We defined suspected gastrointestinal anthrax as acute-onset diarrhoea or vomiting in a resident of Ibanda District during August 12–30, 2022. Confirmed cases were those suspected cases with a clinical sample that tested positive for Bacillus anthracis by culture or PCR. We reviewed health facility records to collect data on cases. We conducted a retrospective cohort study including all households potentially exposed to an anthrax-infected animal during this period and used log-binomial regression to identify risk factors.
Results: There were 45 suspected cases (1 fatal) and 1 confirmed case. Twenty-seven (60%) of these cases were males; median age was 27 (IQR 12-45) years. Case-patients presented with abdominal pain (96%), vomiting (60%), and non-bloody diarrhoea (57%). Compared to unexposed cohort members, persons who ate (RR=4.7, 95%CI: 1.8-12.6) or ate and prepared the meat (RR=4.9, 95%CI: 1.3-13.5) were at increased risk of anthrax infection.
Compared to those who ate only boiled meat, those who ate only roasted meat (RR=2.7, 95%CI: 1.1-6.2) or fried and roasted meat (RR=2.8, 95%CI:1.2-6.7) were at increased risk. Twenty-five (89%) case-patients who provided information on the source of meat, purchased meat from Butcher A. Interviews revealed that Butcher A had obtained meat from a cow that had died suddenly in the neighbouring district of Kazo and sold it in the community below the normal price.
Conclusion: Despite guidelines preventing butchering or eating animals that die suddenly, communities continue this practice, leading to anthrax outbreaks. Continued sensitization of the community on the health risks of eating meat from animals that have died of unknown causes, regular meat inspection, and annual vaccination of animals against anthrax in Ibanda and neighbouring districts could reduce the anthrax burden in Uganda.
Background
Anthrax is a zoonotic disease caused by the spore-forming bacterium Bacillus anthracis (1). Animals get infected by breathing or ingesting Bacillus anthracis spores from contaminated soil, plants or water. Human infections usually occur following direct or indirect contact with infected animals or occupational exposure to infected or contaminated animal products(2). In humans, anthrax presents in three main forms: cutaneous, gastrointestinal and inhalational.
Cutaneous anthrax occurs when spores enter the body through skin lesions, inhalational anthrax when spores are inhaled, and gastrointestinal anthrax when spores are ingested. Gastrointestinal anthrax is the rarest form, occurring in 1% of human anthrax cases (3). This form is commonly naturally obtained through eating raw or undercooked meat from an infected animal. The incubation period for the gastrointestinal form is usually 2 to 7 days, but may be as short as 15 hours(4). Without antibiotics, the case fatality rate for gastrointestinal anthrax could be 25-75% with higher rates observed among children(5).
Anthrax is both enzootic and endemic in many African countries, with several outbreaks reported among wildlife, domestic livestock, and human populations in East Africa(6-8). Uganda has previously experienced numerous human outbreaks, especially in districts characterized by dryland savannah vegetation located in the “cattle corridor”. These include: Kween (9), Arua (10) Isingiro (11) and Kiruhura (12). Specifically, in the Western region, previous human outbreaks were only reported in Kiruhura and Isingiro Districts. These re-occurring anthrax outbreaks increase the risk to global health security, with a potential of spread to other parts of the world and increasing potential of anthrax bioterrorism.
On August 23rd 2022, the Uganda Ministry of Health received an alert of a death and illness due to an “unknown” disease in Katooma II and Kisiita Villages in Kagongo Division Ibanda Municipality. The “unknown’ disease was characterized by diarrhoea, vomiting, headache, general body weakness, abdominal pain, and nausea among patients. Laboratory investigations of human samples collected from reported cases indicated anthrax. Since no documented anthrax outbreak had previously been reported in Ibanda District, we investigated the human anthrax outbreak to establish the scope of the problem, identify possible exposures for infection, and recommended evidence-based control and prevention interventions.
Methods
Outbreak area
The outbreak took place in Ibanda District (Figure. 1), located in the pastoral range lands of Southwestern Uganda. Its approximate total population was 277,300 (UBOS, 2021). Major economic activities in the area are livestock and farming. Ibanda District borders Mbarara District to the south, Kazo to the East, Buhweju to the West, and Kamwenge to the North all of which are located in the pastoral range lands of Southwestern Uganda.
Case definitions and finding
We defined a suspected case as an acute onset of ≥2 of the following symptoms; fever, anorexia, abdominal pain, vomiting, diarrhoea (bloody/non-bloody), and sore throat, that occurred between August 12 – 30, 2022, in a resident of Ibanda Municipality. A Confirmed was defined as a suspected case-patient with laboratory confirmation of Bacillus anthracis by PCR from a clinical sample.
Following the above case definitions, we reviewed patient records in private and public health facilities in Ibanda Municipality to identify cases. We developed a line list with patient clinical presentation, person, place, and time characteristics. Additionally, Village Health Teams (VHTs) were asked to notify any cases identified in the community.
Descriptive epidemiology
We described case-patients by person, place, time, and clinical presentation. Using the 2022 municipality projected population data for the wards, we calculated attack rates (AR) by age and sex. We also constructed maps depicting the case-patients’ village centers.
We used an epidemiological curve to describe the course of the outbreak over time. To estimate the start date, we counted back the minimum incubation period (from the date of illness onset of the first case) or median incubation period (from the peak of the epidemic curve), selecting the earlier date.
Hypothesis generation interviews
Using a Kobocollect tool, we interviewed 19 individuals in the community to obtain information about the likely exposures (Occupation, eating beef, handling animals, preparation methods).
Retrospective cohort study
We conducted a retrospective cohort study in Kagongo Parish, which had the highest attack rates. All members in the community had a possible exposure to the contaminated meat. To collect demographic data, including age, sex, place of residence, and occupation, as well as data on potential exposures (eating beef, handling animals, and preparation methods), we used a structured questionnaire. We defined the effective exposure period to be 1-7 days before the onset of symptoms, which is the time frame between minimum and maximum incubation period of gastrointestinal Anthrax.
We collected data using Kobo Toolbox and managed it in Microsoft Excel. We then performed basic analysis on frequencies and proportions in Epi Info 7.2. Using a modified Poisson regression, we computed risk ratios to establish the risk factors associated with anthrax infection.
Traceback and environmental assessment findings
We interviewed case-patients, vendors of roasted meat, and butchers to identify the possible sources of the contaminated meat. We observed the meat selling points, food vendors and food hygiene practices. We visited the farms in Kazo and Ibanda District where the meat was obtained.
Laboratory analysis
A total of nine blood samples were collected from human suspects and sent to UVRI for PCR analysis. Seven of the nine samples were from suspects who had been initiated on antibiotic treatment.
Results
Descriptive epidemiology
We identified a total of 45 cases (44 suspects, 1 confirmed case), an overall attack rate of 12/10,000 and one death, a case fatality of 2/100 infected population during the outbreak. Most of the cases (60%) were males. The higher proportion of individuals were aged <35 years (40%) in comparison to <10 (16%), 11-24 (27%), 25-35(18%). More than half of the cases (64%) had primary school as their highest level of education while the rest had secondary education (11%), tertiary education (2%) and others had not had any education (22%).
The cases mainly presented with abdominal pain (96%), vomiting (60%), and non-bloody diarrhoea (57%). No cases presented with skin reddening, swelling, vesicles, itching or an eschar (Figure 3)

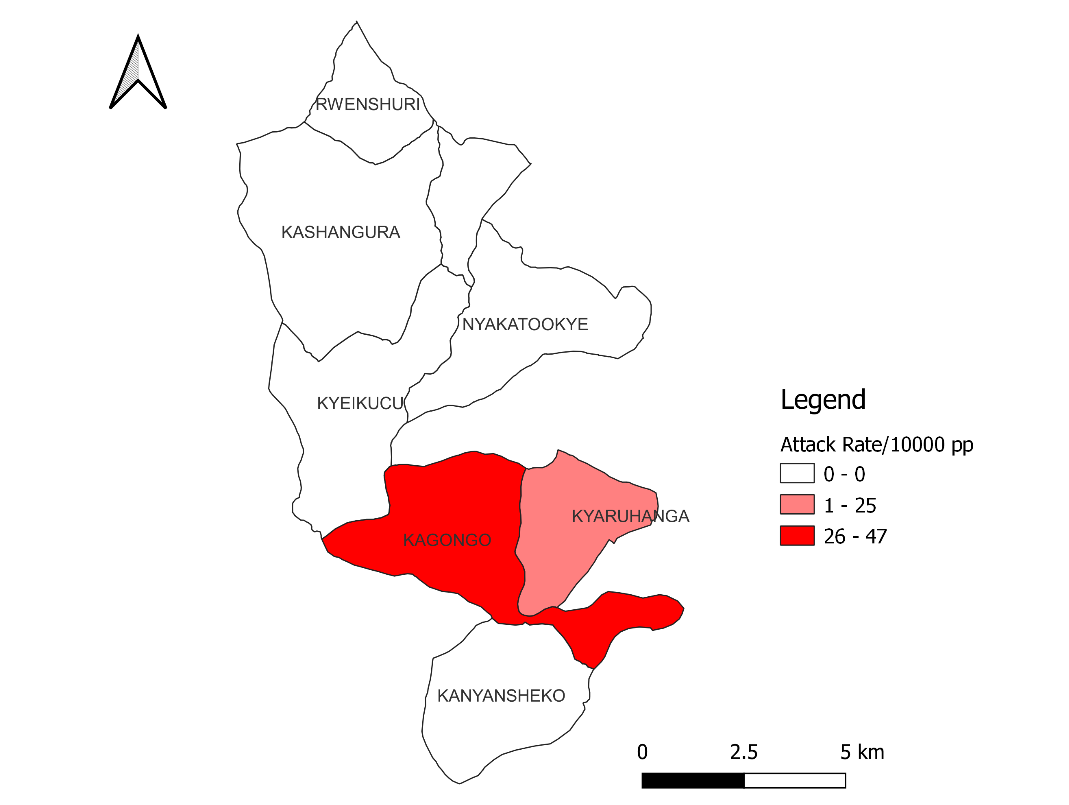
Kagongo division had the highest attack rate (47/10,000 population) in comparison with Kyangura (7/10,000 population). There were no cases recorded in Rwenshuri, Kyeikucu, Nyakatookye, and Kanyansheko (Figure. 2).
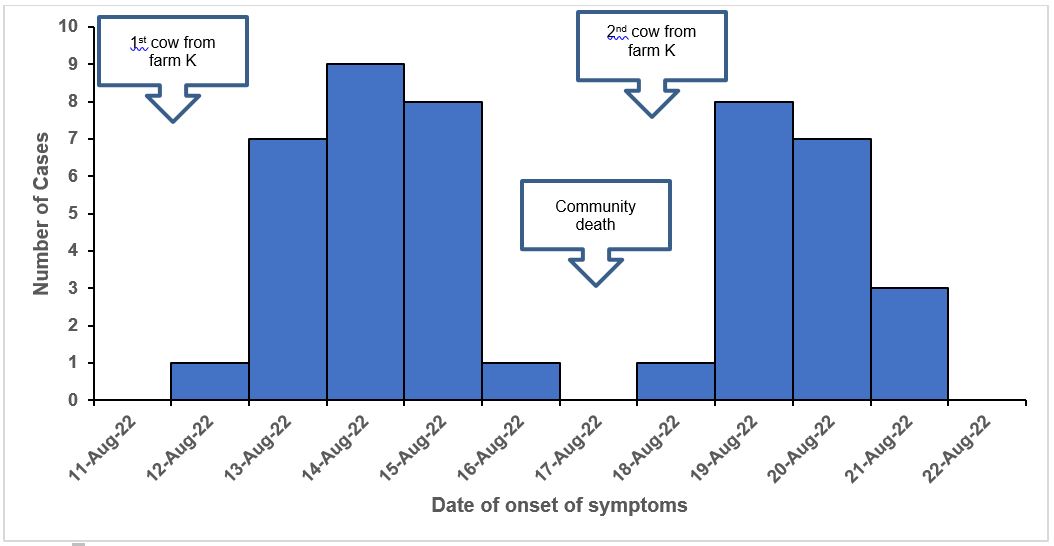
The first cases were reported on August 12, 2022. This was the same day, beef from a cow that had died suddenly at farm K was distributed in the community. Cases were seen to spike up from August 13 and a rapid decline from August 15 to 16, 2022. On August 17, 2022, there was a community death of a 65-year-old male butcher who was linked to the consumption of meat from a cow bought from farm K. No new additional cases were observed on this day. Additional cases were reported on the 18th of August, the same day a second cow that died suddenly was brought from the same farm. Cases rapidly increased on the 19th of August followed by a decline. The epidemic curve suggests a point source exposure from August 12 to 15, 2022 (Figure. 4)
Traceback and environmental assessment findings
Twenty-five (89%) case-patients who provided information on the source of meat, purchased meat from Butcher A. Although there were several butcher shops in Kagongo, Butcher A was the biggest and most popular. Butcher A also supplies meat to street vendors of roasted meat in Kagongo and the neighboring villages. In an interview with butcher A, he revealed that he often bought meat from the Town Council abattoir and local farmers in Kazo.
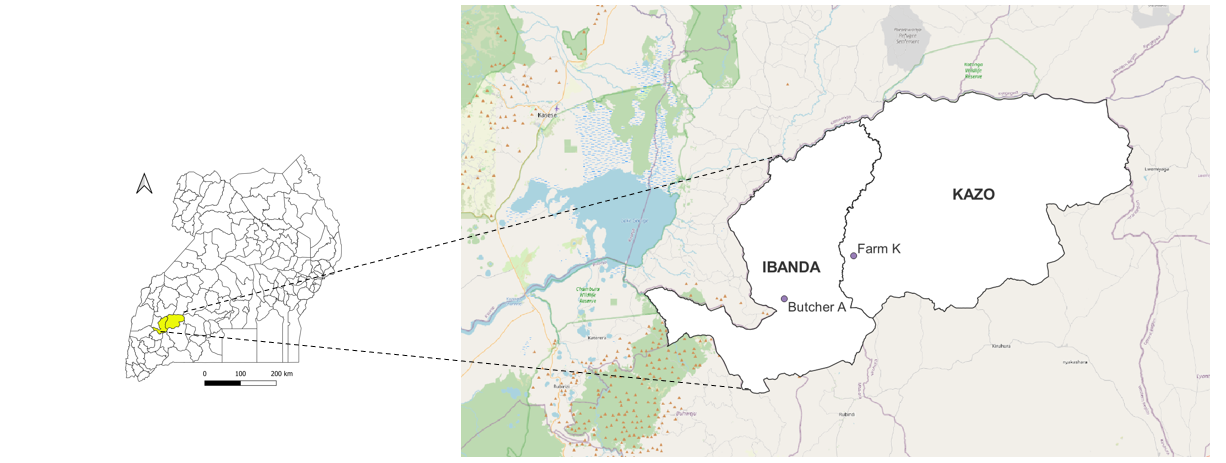
On August 11, 2022, a farmer in Kazo District called butcher A and informed him of an animal that was weak due to an unknown ailment which he wanted to sell off. On August 12 2022, he travelled to Farm K in Kazo District, a neighboring district (Figure 5) where he found that the cow had died suddenly and had been bled out by the farm owner. Butcher A bought the carcass at UGX. 300000 ($78). The animal was slaughtered and skinned at the farm and the meat was transported back to his Butcher in Kagongo, Ibanda Municipality. The beef was sold at half the normal
price (UGX. 6000/Kg Vs full price UGX. 14000/kg), and all the beef got sold out on the same day. He received another phone call on the August 17, 2022 from the same farmer in Ibanda who offered to sell him a second cow at a cheap price UGX. 350000 ($95). On 18th August, he travelled to farm K where he found the cow had already been slaughtered and skinned. Further interviews with the owner of Farm K about the animal did not yield any further details about the animals he had sold to Butcher A.
Further investigations with the roast meat food vendors indicated that they often got meat from Butcher A since his meat was cheap. They seasoned the meat and roasted it for a maximum of 30 minutes. However, on days with high volumes of customers they roasted the meat for less time as the customers were impatient.
Environmental assessments conducted in Kazo where the implicated meat was obtained indicated that there were reports of sudden deaths of animals in the past two weeks. This was observed in communal grazing and drinking areas. Due to the dry season, communal grazing areas were comprised mainly of short grass. Additionally, farmers had been forced to graze their animals on communal grounds.
Hypothesis generation interview findings
None of the case-patients (n=0/19) had contact with a sick animal presenting with anthrax like symptoms, 1/19 case-patients slaughtered a sick animal or animal that had died suddenly, 1/19 skinned and removed organs, 3/19 carried meat, 17/19 ate, while 9/19 prepared the meat. Based on the descriptive epidemiology and hypotheses generation interviews, we hypothesized that the outbreak could have been associated with eating, preparing, carrying or skinning meat from a cow that died of unknown causes.
Retrospective cohort study results
A total of 139 residents of Kagongo were included in the cohort study. We found that compared to unexposed (no contact with the dead animals or their beef) cohort members, persons who ate (RR=4.7, 95%CI: 1.8-12.6) or ate and prepared the meat (RR=4.9, 95%CI: 1.3-13.5) were at an increased risk of becoming a case (Table 2a)
Table 1a: Common group reference analysis of risk factors associated with gastro-intestinal anthrax, Ibanda District, Uganda, August 2022
| Category | Touch /Carried remains | Slaughtered/ Skinned / removed organs | Cooked | Ate | Sick | Total | RR (95% CI) |
| 0 | – | – | – | – | 4 | 40 | REF |
| 1 | + | – | – | – | 0 | 6 | |
| 2 | – | + | – | – | 0 | 0 | |
| 3 | – | – | + | – | 0 | 1 | |
| 4 | – | – | – | + | 27 | 57 | 4.7(1.8-12.6)* |
| 5 | + | + | – | – | 0 | 1 | |
| 6 | + | – | + | – | 0 | 1 | |
| 8 | – | – | + | + | 11 | 22 | 4.9(1.3-13.5)* |
| 9 | + | – | – | + | 1 | 6 | 1.7(0.2-12.5) |
| 10 | + | – | + | + | 2 | 5 | 4.0(0.9-16.5) |
– No, + Yes, *Statistically significant with 95% CI not including one and p<0.05
Additionally, compared to those who only ate boiled meat, those who ate only roasted meat (RR=2.7, 95%CI: 1.1-6.2) or fried and roasted meat (RR=2.8, 95%CI:1.2-6.7) were at increased risk of becoming a case (Table 2b).
Table 2b: Common group reference analysis of risk factors associated with gastro-intestinal anthrax, Ibanda District, Uganda, August 2022
| Category | Fried | Boiled | Roasted | Smoked | Sick | Total | |
| 0 | – | + | – | – | 4 | 13 | REF |
| 1 | + | – | – | – | 1 | 1 | |
| 2 | – | – | + | – | 20 | 24 | 2.7(1.2-6.2)* |
| 3 | – | – | – | + | 0 | 1 | |
| 4 | + | + | – | – | 0 | 3 | |
| 5 | – | + | + | – | 2 | 22 | 0.3(0.1-1.4) |
| 6 | – | + | – | + | 1 | 4 | 0.8(0.1-5.3) |
| 7 | + | – | + | – | 7 | 8 | 2.8(1.2-6.7)* |
| 8 | + | – | – | + | 0 | 1 | |
| 9 | – | – | + | + | 3 | 3 | |
| 10 | + | + | + | – | 1 | 5 | 0.6(0.1-4.5) |
| 11 | + | + | – | + | 0 | 4 |
– No, + Yes, *Statistically significant with 95% CI not including one and p<0.05
Discussion
This was a gastrointestinal anthrax outbreak associated with eating roasted meat, or a combination of fried and roasted meat from animals that died suddenly. The implicated meat was obtained from animals that died suddenly from a farm (unlicensed source) in the neighboring District of Kazo and sold in the local Kagongo community.
This outbreak was associated with eating meat obtained from animals that had died of unknown cause suggesting a gastrointestinal anthrax disease(13). Additionally, case-patients presented with mainly gastrointestinal symptoms further pointing to gastrointestinal anthrax disease. Although this form is rare, Uganda has experienced a couple of these in the recent past, particularly in 2017(11) and 2018(9) in the Southwestern and Eastern regions. In comparison to another solely gastrointestinal anthrax outbreak in Southwestern Uganda, this outbreak had a higher attack rate (14 per 10,000 in the current outbreak compared to 1.2 per 10,000). This could be linked to the multiple sources of meat from animals that had died of unknown causes.
Although the documented gastrointestinal anthrax fatality rate is 25-75%, our study found a lower case fatality rate of 2.2% (1/44) with no hospitalizations(14). By the time of our investigation, 64% of the case-patients reported receiving an antibiotic treatment following onset of symptoms. Gastrointestinal anthrax typically presents similar to other gastrointestinal illnesses commonly treated with antibiotics(14). Previous anthrax outbreaks(9, 12) in Uganda found patients had taken antibiotics by the time of the outbreak confirmation, which may have improved patient outcomes.
In this outbreak eating roasted meat or a combination of fried and roasted meat increased the risk of suffering from gastrointestinal anthrax. A previous study found that roasting was not effective in killing bacillus anthracis spores(15).
The implicated meat was obtained from animals that died suddenly on a farm (unlicensed source) in the neighboring District of Kazo and sold in the local Kagongo community. Kazo, had previously experienced anthrax outbreaks in 2018 (16). Anthrax spores can persist in the soil for decades (17), increasing the likelihood of future outbreaks. This outbreak occurred in late August, a drought season characterized by short grass(18), forcing animals to graze closer to the soil. No animal deaths were reported in the area (Kagongo) where the outbreak occurred suggesting that the infected meat was imported.
Further investigations found that a butcher brought meat from in Kazo District and distributed it in the affected village (Kagango). It is therefore not surprising that no forms of cutaneous forms of anthrax, which are commonly linked to processing meat and handling animal carcasses, were observed (19). Since this meat was obtained from an unlicensed source (farm), the butcher had to sell the meat cheaply to ensure that it is sold out on the same day without the local authorities’ knowledge.
Limitations
Our findings should be interpreted with the following limitations. During this investigation, we were unable to obtain beef samples for confirmation of Bacillus anthracis, as the implicated meat had already been consumed. Additionally, there could have been more cases with mild symptoms that were either missed or linked to other gastrointestinal illnesses, thus underestimating the actual scope of the outbreak. Furthermore, we could not have a detailed investigation of the deceased animals as the farm owners were unwilling to be interviewed hence missing on information such as the source of the implicated animals among other critical information for the response. Lastly, no animal sample was tested to confirm anthrax in animals since the farm owners cleared all the animal remains.
Conclusion
Our investigation revealed a human gastrointestinal anthrax outbreak in Ibanda District attributed to eating roasted meat or a combination of fried and roasted from a cow that died suddenly. To address this, the Ibanda District should engage the municipality and respective authorities to enforce guidelines in the public health act (Cap. 281), cattle traders act (Cap.43), and the Food and Drugs Authority act (SI. 278) on inspection, licensing, and distribution of animal products in the community. Additionally, the regional veterinary representatives should sensitize and encourage farmers on the importance of vaccinating animals against anthrax in Ibanda and neighboring Districts.
Public health actions
Following our findings, the Ibanda District Local Government passed a by-law prohibiting the sale of meat that has not undergone proper meat inspection and the sale of meat in unlicensed premises. The District Veterinary and District Health team conducted community sensitization meetings on how to prevent and control anthrax both in humans and animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors appreciate the Ministry of Health for providing authorization to carry out this investigation in Ibanda District. We also appreciate the technical support provided by the Uganda National Institute of Public health. The authors also so wish to thank the office of the District Health officer Ibanda for the support rendered during community engagements and collection of data. Finally, we thank the US-CDC for supporting the activities of the Uganda Public Health Fellowship Program (UPHFP).
Copyright and licensing
All materials in the Uganda Public Health Bulletin is in the public domain and may be used and reprinted without permission; citation as to source; however, is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J Med. 1999;341(11):815-26.
- Doganay M, Demiraslan H. Human anthrax as a re-emerging disease. Recent Pat Antiinfect Drug Discov. 2015;10(1):10-29.
- Kamal SM, Rashid AK, Bakar MA, Ahad MA. Anthrax: an update. Asian Pac J Trop Biomed. 2011;1(6):496-501.
- Maddah G, Abdollahi A, Katebi M. Gastrointestinal anthrax: clinical experience in 5 cases. Caspian journal of internal medicine. 2013;4(2):672.
- Misgie F, Atnaf A, Surafel K. A review on anthrax and its public health and economic importance. Acad J Anim Dis. 2015;4(3):196-204.
- Mwakapeje ER, Høgset S, Fyumagwa R, Nonga HE, Mdegela RH, Skjerve E. Anthrax outbreaks in the humans – livestock and wildlife interface areas of Northern Tanzania: a retrospective record review 2006–2016. BMC Public Health. 2018;18(1):106.
- Gachohi J, Gakuya F, Lekolool I, Osoro E, Nderitu L, Munyua P, et al. Temporal and spatial distribution of anthrax outbreaks among Kenyan wildlife, 1999–2017. Epidemiology & Infection. 2019;147.
- Driciru M, Rwego IB, Asiimwe B, Travis DA, Alvarez J, VanderWaal K, et al. Spatio-temporal epidemiology of anthrax in Hippopotamus amphibious in Queen Elizabeth Protected Area, Uganda. PloS one. 2018;13(11):e0206922-e.
- Kisaakye E, Ario AR, Bainomugisha K, Cossaboom CM, Lowe D, Bulage L, et al. Outbreak of Anthrax Associated with Handling and Eating Meat from a Cow, Uganda, 2018. Emerg Infect Dis. 2020;26(12):2799-806.
- Ntono V, Eurien D, Bulage L, Kadobera D, Harris J, Ario AR. Cutaneous anthrax outbreak associated with handling dead animals, Rhino Camp sub-county: Arua District, Uganda, January–May 2018. One Health Outlook. 2021;3(1):8.
- Nakanwagi M, Ario AR, Kwagonza L, Aceng FL, Mwesigye J, Bulage L, et al. Outbreak of gastrointestinal anthrax following eating beef of suspicious origin: Isingiro District, Uganda, 2017. PLOS Neglected Tropical Diseases. 2020;14(2):e0008026.
- Musewa A, Mirembe BB, Monje F, Birungi D, Nanziri C, Aceng FL, et al. Outbreak of cutaneous anthrax associated with handling meat of dead cows in Southwestern Uganda, May 2018. Trop Med Health. 2022;50(1):52.
- Sirisanthana T, Brown AE. Anthrax of the gastrointestinal tract. Emerg Infect Dis. 2002;8(7):649-51.
- Bravata DM, Holty JE, Wang E, Lewis R, Wise PH, McDonald KM, et al. Inhalational, gastrointestinal, and cutaneous anthrax in children: a systematic review of cases: 1900 to 2005. Arch Pediatr Adolesc Med. 2007;161(9):896-905.
- Pamartha AP, Lazuardi M, Harijani N, Soelih AT, Estoepangestie DH, Tacharina MR, et al. The effect of cooking methods to the existence of bacillus sp. Spores in beef. 2019.
- Migisha R, Mbatidde I, Agaba DC, Turyakira E, Tumwine G, Byaruhanga A, et al. Risk factors for human anthrax outbreak in Kiruhura District, Southwestern Uganda: a population-based case control study. 2021.
- World Health O. Anthrax in humans and animals: World Health Organization; 2008.
- TORETI A, BAVERA D, ACOSTA NJ, CAMMALLERI C, DE JA, DI CC, et al. Drought in East Africa August 2022.
- Mwakapeje ER, Høgset S, Softic A, Mghamba J, Nonga HE, Mdegela RH, et al. Risk factors for human cutaneous anthrax outbreaks in the hotspot districts of Northern Tanzania: an unmatched case-control study. R Soc Open Sci. 2018;5(9):180479.


Comments are closed.