Factors associated with Loss to Follow-Up among adults aged 40 years and above living with HIV in Mid-Western Uganda, 2020 ̶ 2022
Robert Zavuga*1, Richard Migisha1, Peter Chris Kawungezi1, Rebecca Akunzirwe1, Daniel Kadobera1, Benon Kwesiga1, Lillian Bulage1, Joshua Kayiwa2, Issa Makumbi2 and Alex Riolexus Ario1 Institutional affiliations: 1Uganda Public Health Fellowship Program, Uganda, National Institute of Public Health, Kampala, Uganda, 2National Public Health Emergency Operations Center, Ministry of Health, Kampala, Uganda Correspondence*: Tel: +256772655723, Email: rzavuga@musph.ac.ug,
Summary
Background: Expanded access to Antiretroviral Therapy (ART) has improved the longevity of persons living with HIV (PLHIV), and continuation of treatment is key to successful treatment outcomes. However, there are limited data on loss to follow-up (LTFU) amongst older PLHIV in Uganda. We determined the occurrence of LTFU and associated factors among adults ≥40 years living with HIV in mid-western Uganda.
Methods: We analyzed data for PLHIV aged ≥40 years submitted to the Inter-agency Collaborative for Program Improvement (ICPI) database from January 2020 ̶ December 2022 in Bunyoro and Tooro sub-regions in mid-Western Uganda. The ICPI database is a national platform to which all US President’s Emergency Plan for AIDS Relief (PEPFAR)-supported partners/agencies involved in HIV care submit aggregated data every three months. The database classifies patients who were ever enrolled on ART as LTFU if they have not returned for refills for >90 days from their last missed appointment and are not reported as dead or transferred to another ART clinic. Multiple logistic regression was conducted to identify factors associated with LTFU.
Results: Among 62,794 PLHIV aged ≥40 years from 2020 ̶ 2022, 35,194 (56%) were female and 41,946 (67%) were aged 40-49 years. In total, 1,508 (2.4%) were LTFU, among whom 814 (54%) were male and 936 (62%) were aged 40-49 years. Being male (adjusted odds ratio [aOR]=1.3; CI: 1.2-4.8), aged ≥50 years (aOR=1.8; CI: 2.0-6.7), and having registered for treatment at a regional referral hospital (versus all lower-level health facilities) (aOR=1.5; CI: 1.4-6.0) were associated with LTFU.
Conclusion: Although LTFU among PLHIV ≥40 years was low during 2020 ̶ 2022, males aged ≥50 years and people who registered for treatment at regional referral hospitals still faced increased odds of LTFU. Targeted efforts that track and follow up PLHIVs in this demographic group who receive treatment from regional referral hospitals could reduce further LTFU.
Introduction and Background
The introduction of Highly Active Antiretroviral Therapy (HAART) has led to improved longevity among persons living with HIV (PLHIV). As a result, the number of older PLHIV has increased in the world today [1]. Globally, there is an estimated 5.7 million PLHIV ≥50 years of age, and the majority live in sub-Saharan Africa (SSA) [2]. The increase in older persons living with HIV (PLHIV) is partly attributed to the accelerated scale-up of access to anti-retroviral treatment (ART), which led to a decline in HIV-related morbidity, mortality, and new HIV infections, shifting the proportion of disease burden from younger adults to older age groups [3].
Older PLHIV are a vulnerable group because they face dual stigma, that is, age-related and HIV-related stigma [4]. Ageism and stereotyping directed towards people as they grow old is likely to cause social discrimination. On the other hand, the HIV-related stigma and discrimination associated with PLHIV causes social exclusion [5]. This in turn affects their adherence to treatment and retention in care and as such leads to a higher risk of disease progression, increased morbidity and mortality [6].
Retention in care of PLHIV is a critical aspect in HIV management programs and as such a loss to follow-up (LTFU) undermines the effectiveness of such interventions and facilitate the onward transmission of the HIV virus. LTFU is a situation where PLHIV who have previously been receiving ART care suddenly become unaccounted for within a specified period [7]. There is evidence that loss to follow up (LTFU) among patients with HIV in care leads to poorer clinical outcomes and higher risks of opportunistic infections and death [8-11]. Despite this, SSA continues to experience sub optimal long-term ART retention rates [12]. In Uganda, the incidence of LTFU from HIV care has been reported to range from 9 to 20% [13, 14]. It is hypothesized that the prevalence may be higher in the advanced-age population where patients may feel stigmatized, discriminated, and lack social support [15].
Although several studies have examined determinants of LTFU broadly among PHLIV, limited attention has been given to the specific sub group of adults aged 40 years and above. Data from a local non-government organisation (NGO) dealing in HIV care and treatment in mid-western Uganda, suggest that the number of lost patients exceeds those enrolled in care by approximately 6,000 per year. However, data on the incidence and attributes to LTFU among older people ≥40 years is limited. We determined the occurrence and determinants of LTFU among adults in ART care ≥ 40 years living with HIV in mid-western Uganda, 2020-2022 to inform control and prevention interventions.
Methods
Study design and setting
We conducted a retrospective ecological study which involved analysis of routinely collected program data on HIV care and treatment submitted to the Interagency Collaborative for Program Improvement (ICPI) data-base from January 2020 ̶ December 2022 in mid-western Uganda.
Mid-Western Uganda comprises Bunyoro and Tooro sub-regions. Bunyoro Sub-region is made up of 9 districts: Buliisa, Hoima city, Hoima, Kagadi, Kakumiro, Kibaale, Kikuube, Kiryandongo and Masindi. Tooro Sub-region is made up of 10 districts: Bundibugyo, Bunyangabu, Fort Portal city, Kabarole, Kamwenge, Kasese, Kitagwenda, Kyegegwa, Kyenjojo and Ntoroko.
HIV care services are provided by the Ministry of Health (MoH) through the ART accredited health facilities. Every ART clinic at a health centre is headed by a medical doctor or clinical officer (who provides diagnosis and treatment), nurses, counsellors (who counsel patients on adherence and other psychosocial needs), a data management team (which does data entry and manages the ART clinic database), and volunteers (who organize the patient files and do patient follow-up).
The ICPI data-base comprises of HIV care data from the 19 districts that make up mid-western Uganda. The ICPI is a platform where all partners/agencies involved in HIV care that are supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR) submit aggregated data. The data is submitted on a quarterly basis (every three months) in an aggregate form without any individual patient identifiers.
All the ART clinics in the region receive donor support from PEPFAR and work with the Ugandan government and a local non-government organisation (NGO) called Baylor Uganda Limited. This support involves scaling up of HIV services through clinical mentorship, training of health care workers, renovation of facilities and technical assistance in monitoring and evaluation.
Generation of HIV surveillance data
Data are collected by clinicians as part of their routine medical care. At the health facility level, patient information from patient medical records is entered into the electronic data base by trained data personnel. It contains personal information like name, age, sex, patient number, marital status, level of education, weight, height, CD4 cell count, date of ART enrollment among others. Routine data quality audits are done every 3 months to ascertain completeness and accuracy. These data are then summarized and submitted to the ICPI data base in aggerate form without individual patient identifiers. Data from different health centers are sent to the district and later merged into regions.
Study variables
The primary outcome variable was LTFU which was defined as failure of a patient enrolled in care to show up after missing treatment for more than one quarter (90 days) after initiation on ART and has not been reported dead or transferred out to another ART clinic [16]. Patients who showed up after being declared LTFU were classified as alive and active on treatment. Patients were classified as dead if they appeared in the data base as dead. Death at health facilities is certified with evidence of a death certificate or through information by next of kin. Patients were also classified as ‘Transferred out’ if they were officially transferred to other ART accredited clinics outside the midwestern region by use of a referral form.
Independent variables included age group, sex, sub-region of residence and health facility level. Heath facility levels included those from the lowest level to the highest level in the region in the hierarchical order [17]. That is, the lowest health centers being HCIIs (found at parish level), followed by HCIIIs (found at sub-county level), HCIVs (found at county/health sub-district level), and district hospitals (found at district level) and the highest health facilities being regional referral hospitals (found at region level).
Data abstraction and analysis
Data were entered into Excel and exported to Stata version 16 software (Stata Corporation, College Station, Texas, USA) for analysis. Different variables for the years of 2020, 2021, and 2022 were captured. These years were considered because we wanted to determine the level of LTFU after the COVID-19 response period (2020 ̶ 2021). A data abstraction form was used to extract information on age-group, sex, sub-region, and level of health facility.
We summarized categorical data like sex, age-group, level of health facility, and subregion by frequencies and percentages. Loss to follow-up was calculated by the number patients LTFU divided by the total number of patients enrolled during the study period and was expressed as a percentage.
Multiple logistic regression analysis was conducted to identify the factors associated with LTFU. In the analysis, LTFU was dichotomized into binary outcome (yes/no) where yes were patients LTFU and no were patients retained in care. The association between independent variables and the outcome variable was presented as odds ratios (ORs) and 95% confidence intervals (CIs).
Ethical Considerations
The study utilized routine surveillance data reported by health facilities in the ICPI platform which is also aggregated with no individual patient identifiers. However, permission to access the database was sought from the US Centers for Disease Control and Prevention (CDC) office in Uganda and the Ministry of Health. The Center for Global Health, US Center for Disease Control and Prevention also determined that this activity was not human subject research and its primary intent was for public health practice or disease control. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy. §§See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq. The study team did not interact with any human subjects and all the data were de-identified at health facilities and obtained as aggregate data in the data base. All the investigators did not have any access to individual patient information. Data were only accessed by the study team.
Results
Loss to follow-up amongst persons living with HIV, mid-western Uganda, January 2020 ̶ December 2022
We abstracted 62,794 patients’ records, of which 35,194 (56%) were female, 41,946 (67%) were between age group 40-49 years and 36,546 (58%) were from Tooro region.
Among the patients enrolled in the study within the review period, 60,646 (96.6%) remained in care while 1,508 (2.4%) were LTFU, 377 (0.6%) died, and 263 (0.4%) were transferred out of the region (Figure 1).
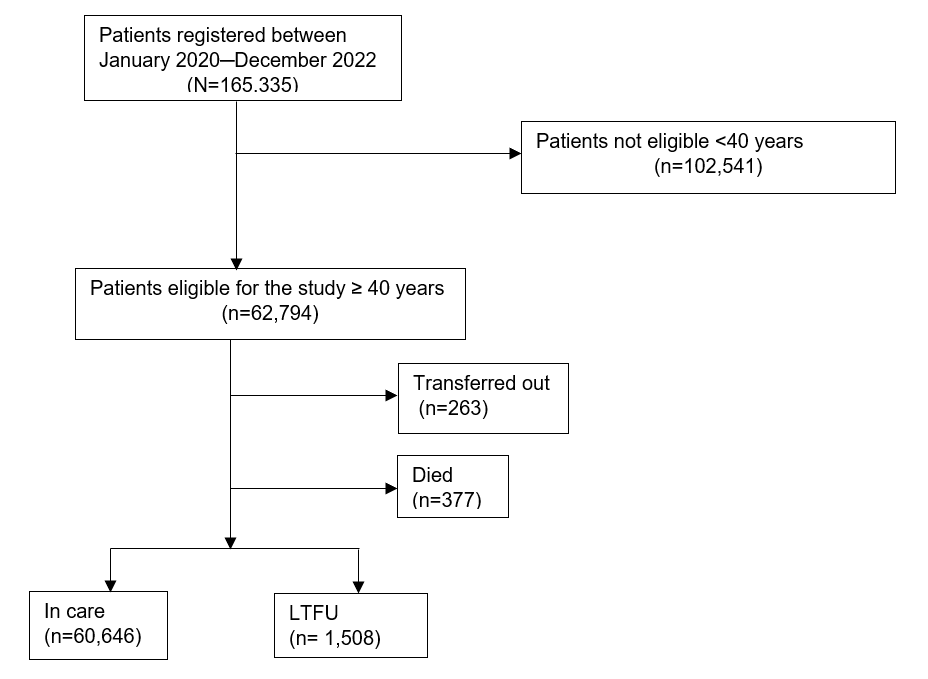
Characteristics of persons living with HIV who were lost to follow-up, mid-western, Uganda, January 2020 ̶ December 2022
Among those LTFU, 814 (54%) were male, majority 936 (62%) were in the 40-49 age group, and 824 (55%) were from Health Center IIIs (Table 1).
Table 1: Characteristics of persons living with HIV who were lost to follow-up, mid-western, Uganda, January 2020 ̶ December 2022, (N=1,508)
| Characteristic | Number | (%) |
| Sex | ||
| Male | 814 | (54) |
| Female | 694 | (46) |
| Age group | ||
| 40-49 | 936 | (62) |
| ≥50 | 572 | (38) |
| Sub-region | ||
| Tooro | 879 | (58) |
| Bunyoro | 629 | (42) |
| Health facility level | ||
| Regional Referral | 118 | (8) |
| District hospital | 137 | (9) |
| Health Center IV | 366 | (24) |
| Health Center III | 824 | (55) |
| Health Center II | 63 | (4) |
Factors associated with loss to follow-up amongst persons living with HIV from January 2020 ̶ December 2022 in mid-western, Uganda (N=62,154)
At bivariate analysis, sex, age, and higher-level health facility were statistically significant while subregion was not.
In multivariable analysis, being male (adjusted odds ratio [aOR]=1.3; CI: 1.2-4.8), being aged ≥50 years (aOR=1.8; CI: 2.0-6.7), and having registered for treatment at a regional referral hospital (versus all lower-level health facilities) (aOR=1.5; CI: 1.4-6.0) were associated with LTFU (Table 2).
Table 2: Factors associated with loss to follow-up amongst persons living HIV, mid-western, Uganda, January 2020 ̶ December 2022, (N=62,154)
| Characteristic | Lost to Follow-up | Bivariate analysis | Multivariate analysis | |||||
| Yes (n=1,508) | No
(n=60,646) |
OR (95%CI) | P Value | Adjusted OR (95%CI) | P Value | |||
| n | (%) | n | (%) | |||||
| Sex | ||||||||
| Male | 814 | (54) | 27,282 | (45) | 2.6(1.2-6.4) | 0.028 | 1.3(1.2-4.8) | 0.032 |
| Female | 698 | (46) | 33,364 | (55) | Ref | Ref | ||
| Age in years | ||||||||
| 40-49 | 936 | (62) | 40,938 | (67) | Ref | Ref | ||
| ≥50 | 572 | (38) | 19,708 | (33) | 2.4 (1.1-5.4) | <0.001 | 1.8(2.0-6.7) | <0.001 |
| Sub-region | ||||||||
| Tooro | 879 | (58) | 32,140 | (53) | Ref | Ref | ||
| Bunyoro | 629 | (42) | 28,506 | (47) | 1.3(0.2-1.5) | 0.941 | 1.1(0.6-4.7) | 0.502 |
| Health facility level | ||||||||
| Regional referral | 118 | (8) | 9,092 | (15) | 2.7 (1.2-4.7) | 0.011 | 1.5 (1.4-6.0) | 0.037 |
| District hospital | 137 | (9) | 16,956 | (28) | 1.5 (1.7-6.6) | 0.018 | 2.2 (1.4-8.1) | 0.027 |
| Health Center IV | 366 | (24) | 9,096 | (15) | 2.7 (0.9-14.1) | 0.276 | 1.6 (0.2-1.8) | 0.076 |
| Health Center III | 824 | (55) | 22,465 | (37) | Ref | Ref | ||
| Health Center II | 63 | (4) | 3,037 | (5) | 3.0 (0.7-2.9) | 0.077 | 0.4 (0.6-9.7) | 0.151 |
Discussion
We assessed the occurrence of LFTU and the associated factors amongst PLHIV ≥40years enrolled in care from January 2020 ̶ December 2022 in mid-western, Uganda. The occurrence of LTFU among adult PLHIV in care was determined at 2 out of 100 persons. LTFU was associated with being male, being aged ≥50 years, and having registered for treatment at a regional referral hospital (versus all lower-level health facilities). This study provides an insight on the occurrence of LTFU amongst the PLHIV ageing population in mid-western Uganda.
The occurrence of LTFU amongst PLHIV ≥40years in ART care was found to be approximately 2 in 100 individuals. This is low compared to what was reported in other studies. A retrospective study that was conducted in central Uganda in 2019 which also analyzed routinely collected HIV care and treatment program data from 2014─2016 reported a cumulative LTFU incidence of 7.5 per 100 person years[18]. The authors attributed the high incidence of LTFU to limited counselling and patient support services. Similarly a study done in Zimbabwe reported an incidence rate of 5.8 per 100 person years[19]. The reason for low LTFU in our study could be attributed to patient support services that involve tracking of patients enrolled in care and following up on those that have missed their appointments or missed drug refills [20, 21]. Keeping a record of patients in ART care and tracking them is an effective way of identifying individuals who are at risk of LTFU and helps to intervene in a timely manner. Low LTFU is generally a good outcome as it indicates that a higher proportion of individuals is adhering and retained in care.
Although the study had a higher number of females, males were more likely to be LTFU as compared to females. The reason is probably because women have a better health seeking behavior and are more likely to take care of themselves once sick [22, 23]. Another reason for higher LTFU in males than females could be that men may experience HIV-related stigma and discrimination and as such less likely to disclose their HIV status to health workers and or family members [24]. This fear of disclosure and stigma can contribute to nonadherence to treatment and lead to LTFU. Findings of this study are similar to other studies which have reported higher LTFU among men [25-27]. It is important to generate gender specific based interventions aimed at increasing the retention of older men in care.
Despite the fact that the proportion of older persons in our study was low, we observed that they were at a higher risk of LTFU than younger persons. This is probably because of poor access to healthcare by this age group due to unavailability of specialized geriatric clinics in HIV care [28-30]. The unavailability of geriatric services creates barriers for older patients hence leading to missing appointments and dropping away from care. It is important to establish geriatric friendly services in HIV care clinics and as well as establish mechanisms of tracking and following up this demographic group with an aim of retention in care. Geriatric friendly services may include incorporation of treatment of non-communicable diseases (NCDs) treatment in ART clinics. Advanced age is a risk factor of developing NCDs especially diabetes and hypertension and this risk is higher amongst older PLHIV[31]. Studies about the integration of NCD care with HIV care in Uganda have yielded positive results including high retention in care and better clinical outcomes [32, 33]. It is therefore important to integrate NCD care in HIV care and treatment as a way of offering geriatric friendly services.
Participants who received treatment from higher level administrative health facilities like the regional referral hospital and district hospitals were more likely to be LTFU when compared to those that received treatment from lower-level health facilities like HCIV and HCIII. This is possibly because higher level facilities tend to be located in urban centres, experience high patient volumes and have longer waiting times [34]. As result, health workers are less likely to engage patients at a more personal level and this reduces patient engagement, interpersonal communication and subsequent follow-up. These findings are consistent with a study which conducted in Zimbabwe which showed that higher level health facilities experienced more occurrences of LTFU [19]. The authors in this study argued that some HIV patients could seek treatment from areas far away from their places of residence (which are usually high-level health facilities located in urban centres) due to fear of stigma and discrimination. This increases on the patient load experienced at these facilities and hence leading to inadequate care including follow-up. It is important that interventions addressing stigma and discrimination at community level be strengthened and encourage uptake of ART services at lower-level health facilities.
Study limitations and strengths
We acknowledge some limitations in our study. First this was an ecological study which looked at aggregated population-level data rather than individual-level data. There was unavailability of some key demographic, clinical and laboratory information and other variables that could predict LTFU like weight, level of education, marital status, CD4 cell count, and presence of commodities. As a result, we could not establish casual relations to account for individual factors that may have influenced the outcomes. Secondly, this being a retrospective study, it entirely relied on historical data collected from a routine clinical care database. There could have been challenges of data accuracy and completeness recorded during data entry from patient records into the database. Despite these challenges, we utilized routinely collected data from the HIV care and treatment program which undergoes periodic data quality audits. The study includes data from a large sample of 19 districts in mid-western Uganda. This large sample size improves the statistical power and enhances the generalizability of our findings.
Conclusion
Our findings suggest that LTFU among adult PLHIV who are ≥40 years was low. LTFU was associated with being male, being aged ≥50years, and receiving treatment from a higher-level health facility. This study provides an insight on the occurrence of LTFU and associated factors amongst PLHIV ageing population which the MoH can use to improve adherence and retention in care in this age group. We recommended strengthening of the existing systems like tracking and proactive follow-up of patients in order to further reduce the LTFU occurrences. Additionally, creation of interventions and initiatives that address stigma and discrimination amongst older men could improve their engagement in care. Finally, we recommend establishment of geriatric friendly services at ART centres including integration of NCDs in HIV care.
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ contributions
RZ: participated in the conception, design, analysis, interpretation of the study and wrote the draft bulletin; RM, PCK, RA, DK, BK and LB reviewed the report, reviewed the drafts of the bulletin for intellectual content and made multiple edits to the draft bulletin; RM, DK, BK, LB, JK, IM, and ARA reviewed the final bulletin to ensure intellectual content and scientific integrity. All authors read and approved the final bulletin.
Acknowledgements
The authors thank the staff of the Uganda Public Health Fellowship Program for the technical support and guidance offered during this study. The authors also extend their appreciation to the Baylor Uganda team for the support they offer to the various ART centers in health facilities in mid-western Uganda. The authors appreciate the several data management teams in the ART centers at health facilities who ensured reporting of HIV program data from their respective districts, as well as the team managing the ICPI platform; your hard work enabled the availability of data we used for this analysis. Finally, we thank the US-CDC for supporting the activities of the Uganda Public Health Fellowship Program.
Copyright and licensing
All materials in the Uganda Public Health Bulletin are in the public domain and may be used and reprinted without permission; citation as to source; however, is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- UNAIDS DATA 2021 [https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf]
- Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P: Global and regional trends of people living with HIV aged 50 and over: Estimates and projections for 2000–2020. PloS one 2018, 13(11):e0207005.
- Mahy M, Autenrieth CS, Stanecki K, Wynd S: Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS (London, England) 2014, 28(4):S453.
- Brennan-Ing M, Mattas E: Aging with HIV: Working to Ensure Equity and Inclusion. Gerontology 2023.
- Ziersch A, Walsh M, Baak M, Rowley G, Oudih E, Mwanri L: “It is not an acceptable disease”: A qualitative study of HIV-related stigma and discrimination and impacts on health and wellbeing for people from ethnically diverse backgrounds in Australia. BMC Public Health 2021, 21(1):1-15.
- Bernard C, Balestre E, Coffie PA, Eholie SP, Messou E, Kwaghe V, Okwara B, Sawadogo A, Abo Y, Dabis F: Aging with HIV: what effect on mortality and loss to follow-up in the course of antiretroviral therapy? The IeDEA West Africa Cohort Collaboration. HIV/AIDS (Auckland, NZ) 2018, 10:239.
- Mirzazadeh A, Eshun-Wilson I, Thompson RR, Bonyani A, Kahn JG, Baral SD, Schwartz S, Rutherford G, Geng EH: Interventions to reengage people living with HIV who are lost to follow-up from HIV treatment programs: A systematic review and meta-analysis. PLoS medicine 2022, 19(3):e1003940.
- Siril HN, Kaaya SF, Smith Fawzi MK, Mtisi E, Somba M, Kilewo J, Mugusi F, Minja A, Kaale A, Todd J: CLINICAL outcomes and loss to follow-up among people living with HIV participating in the NAMWEZA intervention in Dar es Salaam, Tanzania: a prospective cohort study. AIDS research and therapy 2017, 14(1):1-10.
- Kaufmann GR, Elzi L, Weber R, Furrer H, Giulieri S, Vernazza P, Bernasconi E, Hirschel B, Battegay M, Study SHC: Interruptions of cART limits CD4 T-cell recovery and increases the risk for opportunistic complications and death. Aids 2011, 25(4):441-451.
- Blevins M, Jose E, Bilhete FR, Vaz LM, Shepherd BE, Audet CM, Vermund SH, Moon TD: Two-year death and loss to follow-up outcomes by source of referral to HIV care for HIV-infected patients initiating antiretroviral therapy in rural Mozambique. AIDS research and human retroviruses 2015, 31(2):198-207.
- Teshale AB, Tsegaye AT, Wolde HF: Incidence and predictors of loss to follow up among adult HIV patients on antiretroviral therapy in University of Gondar Comprehensive Specialized Hospital: A competing risk regression modeling. PloS one 2020, 15(1):e0227473.
- Kebede HK, Mwanri L, Ward P, Gesesew HA: Predictors of lost to follow up from antiretroviral therapy among adults in sub-Saharan Africa: a systematic review and meta-analysis. Infectious diseases of poverty 2021, 10(1):1-18.
- Okoboi S, Ding E, Persuad S, Wangisi J, Birungi J, Shurgold S, Kato D, Nyonyintono M, Egessa A, Bakanda C: Community-based ART distribution system can effectively facilitate long-term program retention and low-rates of death and virologic failure in rural Uganda. AIDS research and therapy 2015, 12(1):1-9.
- Namusobya J, Semitala FC, Amanyire G, Kabami J, Chamie G, Bogere J, Jain V, Clark TD, Charlebois E, Havlir DV: High retention in care among HIV-infected patients entering care with CD4 levels> 350 cells/μL under routine program conditions in Uganda. Clinical infectious diseases 2013, 57(9):1343-1350.
- Ruiz EL, Greene KY, Galea JT, Brown B: From surviving to thriving: the current status of the behavioral, social, and psychological issues of aging with HIV. Current Opinion in HIV and AIDS 2022, 17(2):55-64.
- Organization WH: Retention in HIV programmes: defining the challenges and identifying solutions: meeting report, 13-15 September 2011. 2012.
- Ssempiira J, Kasirye I, Kissa J, Nambuusi B, Mukooyo E, Opigo J, Makumbi F, Kasasa S, Vounatsou P: Measuring health facility readiness and its effects on severe malaria outcomes in Uganda. Scientific reports 2018, 8(1):17928.
- Kiwanuka J, Mukulu Waila J, Muhindo Kahungu M, Kitonsa J, Kiwanuka N: Determinants of loss to follow-up among HIV positive patients receiving antiretroviral therapy in a test and treat setting: A retrospective cohort study in Masaka, Uganda. PLoS One 2020, 15(4):e0217606.
- Zingoni ZM, Chirwa T, Todd J, Musenge E: Competing risk of mortality on loss to follow-up outcome among patients with HIV on ART: a retrospective cohort study from the Zimbabwe national ART programme. BMJ open 2020, 10(10):e036136.
- Nkolo EKK, Ejike JC, Sensalire S, Ssali JN, Ddumba I, Calnan J, Gonzalez C, Maina N, Dessie M, Bailey L: Clients in Uganda accessing preferred differentiated antiretroviral therapy models achieve higher viral suppression and are less likely to miss appointments: a cross‐sectional analysis. Journal of the International AIDS Society 2023, 26:e26122.
- Obua C, Kayiwa J, Waako P, Tomson G, Balidawa H, Chalker J, Ross-Degnan D, Wahlstrom R: Improving adherence to antiretroviral treatment in Uganda with a low-resource facility-based intervention. Global health action 2014, 7(1):24198.
- Jespersen S, Hønge BL, Esbjörnsson J, Medina C, da Silva Té D, Correira FG, Laursen AL, Østergaard L, Andersen A, Aaby P: Differential effects of sex in a West African cohort of HIV‐1, HIV‐2 and HIV‐1/2 dually infected patients: men are worse off. Tropical Medicine & International Health 2016, 21(2):253-262.
- Lee HY, Jin SW, Henning-Smith C, Lee J, Lee J: Role of health literacy in health-related information-seeking behavior online: Cross-sectional study. Journal of Medical Internet Research 2021, 23(1):e14088.
- Treves-Kagan S, El Ayadi AM, Pettifor A, MacPhail C, Twine R, Maman S, Peacock D, Kahn K, Lippman SA: Gender, HIV Testing and Stigma: The Association of HIV Testing Behaviors and Community-Level and Individual-Level Stigma in Rural South Africa Differ for Men and Women. AIDS and behavior 2017, 21(9):2579-2588.
- Aliyu A, Adelekan B, Andrew N, Ekong E, Dapiap S, Murtala-Ibrahim F, Nta I, Ndembi N, Mensah C, Dakum P: Predictors of loss to follow-up in art experienced patients in Nigeria: a 13 year review (2004–2017). AIDS Research and Therapy 2019, 16(1):30.
- Dalhatu I, Onotu D, Odafe S, Abiri O, Debem H, Agolory S, Shiraishi RW, Auld AF, Swaminathan M, Dokubo K: Outcomes of Nigeria’s HIV/AIDS treatment program for patients initiated on antiretroviral treatment between 2004-2012. PloS one 2016, 11(11):e0165528.
- Kebede HK, Mwanri L, Ward P, Gesesew HA: Predictors of lost to follow up from antiretroviral therapy among adults in sub-Saharan Africa: a systematic review and meta-analysis. Infectious diseases of poverty 2021, 10:1-18.
- Kiplagat J, Tran DN, Barber T, Njuguna B, Vedanthan R, Triant VA, Pastakia SD: How health systems can adapt to a population ageing with HIV and comorbid disease. The Lancet HIV 2022, 9(4):e281-e292.
- Nawagi F, Söderberg M, Berggren V, Midlöv P, Ajambo A, Nakasujja N: Sociodemographic characteristics and health profile of the elderly seeking health care in Kampala, Uganda. Current Gerontology and Geriatrics Research 2018, 2018.
- Ssensamba JT, Mukuru M, Nakafeero M, Ssenyonga R, Kiwanuka SN: Health systems readiness to provide geriatric friendly care services in Uganda: a cross-sectional study. BMC geriatrics 2019, 19:1-13.
- Moyo M, Musekiwa A: Protocol for updated systematic review and meta-analysis on the burden of non-communicable diseases among people living with HIV in sub-Saharan Africa. BMJ open 2022, 12(5):e055895.
- Bulstra CA, Hontelez JA, Otto M, Stepanova A, Lamontagne E, Yakusik A, El-Sadr WM, Apollo T, Rabkin M, Integration UEGo: Integrating HIV services and other health services: A systematic review and meta-analysis. PLoS medicine 2021, 18(11):e1003836.
- Shayo EH, Kivuyo S, Seeley J, Bukenya D, Karoli P, Mfinanga SG, Jaffar S, Van Hout M-C: The acceptability of integrated healthcare services for HIV and non-communicable diseases: experiences from patients and healthcare workers in Tanzania. BMC health services research 2022, 22(1):655.
- Dowhaniuk N: Exploring country-wide equitable government health care facility access in Uganda. International journal for equity in health 2021, 20(1):1-19.
- Naidoo P, Peltzer K, Louw J, Matseke G, Mchunu G, Tutshana B: Predictors of tuberculosis (TB) and antiretroviral (ARV) medication non-adherence in public primary care patients in South Africa: a cross sectional study. BMC public health 2013, 13:1-10.


Comments are closed.