Assessment of mpox severity, Uganda, November 2024‒February 2025
Authors: Emmanuel Mfitundinda1, Joyce Owens Kobusingye1, Emmanuel Okiror Okello1, Daniel Wenani1, Janet Lubega Kobusinge1, Patrick Kwizera1, Joanita Nalwanga1, Charity Mutesi1, Hannington Katumba1, Loryndah Olive Namakula1, Annet Mary Namusisi1, Bridget Ainembabazi1, Gertrude Abbo1, Richard Migisha1, Patricia Eyu1, Lilian Bulage1, Ivan Lukabwe2, Benon Kwesiga1, Alex Ario Riolexus1 Institutional affiliations: 1Uganda National Institute of Public Health (UNIPH), Uganda Public Health Fellowship Program (UPHFP), 2Uganda National institute of Public Health (UNIPH), Health Informatics Fellowship Program Correspondence*: +256 777 166851, emmamfitundinda@uniph.go.ug
Summary
Background: On July 24, 2024, Uganda’s Ministry of Health confirmed an mpox outbreak, which spread nationwide, with 377 confirmed cases by November 1, 2024. Assessment for severity was suboptimal, yet it is useful for identifying risk factors for severe illness, evaluating efficacy of treatment modalities, and prioritizing care. We assessed for severity and its associated factors among confirmed mpox case-patients, November 2024–February 2025.
Methods: We conducted a cross-sectional study among all confirmed mpox case-patients who were admitted in isolation and treatment units of Entebbe, Nakasongola, and Mbarara, as well as in health facilities in Masindi and Mayuge districts that were most affected at the time. We used the mpox severity scoring system (MPSSS) tool to assess severity. The MPSSS tool is a 7-parameter numerical scoring tool Each parameter is scored on a scale of 0-4; the total score ranges from 0-23. We used a cutoff of ≥7 for severe disease. We used Wilcoxon rank-sum tests to compare the severity scores and background characteristics.
Results: We investigated 244 case-patients. Of these, 128 (52%) were males; the median age was 29 years, interquartile range (IQR)=24-38, range=1day-54years. Of the total participants, 75% (185/244) presented with severe disease, and 30% (74/244) had underlying conditions, with living with HIV (LHIV) being the most common underlying condition (88%, 65/74). Among the participants, 5.7% (14/244) died, with 71% (10/14) of these having underlying conditions. The median severity score was 12.5, interquartile range of 7-16. Mpox case-patients with any underlying illness were more likely to have higher severity scores when compared to those without (p<0.001).
Conclusion: We found a high prevalence of mpox severity, with having an underlying illness increasing the likelihood of severe disease. We recommend prioritizing case-patients with underlying conditions for inpatient care and vaccination to protect them from severe mpox disease.
Background
Mpox is a multisystemic disease affecting several organs of the body and in some cases leading to death. The clinical presentation of the disease differs by the route of exposure, immune status of the host, the strain of the virus and the dose of the virus. Additionally, the disease has variable clinical severity. People living with HIV(PLHIV) especially those with advanced disease, children <5 years of age, and pregnant women are more likely to develop severe disease and have higher case fatality rates. Clade Ib is more likely to cause severe disease compared to clade Ia and II (1). A study in New York City piloted an mpox Severity Scoring System (MPSSS) tool to guide health workers on severity assessment and also assessed validity of the tool. It was found to match with other surrogate measures of severity of the disease and to agree with other monitoring parameters (2).
On July 24, 2024, the Uganda Ministry of Health (MoH) through the National Emergency Operations Centre confirmed an mpox outbreak in Kasese District, Uganda. Mpox cases have since then continued to rise with spread to over 100 districts in the country. Some of the cases have presented in a very sick state. Severity assessment has been suboptimal in the response, yet assessment for severity can be useful in identifying risk factors for severe illness, monitoring disease progression, and in evaluating efficacy of different treatment modalities. We estimated the prevalence of mpox severity and recommend evidence-based measures to prevent or reduce severity related to mpox, Uganda, November 2024‒February 2025.
Methods
Uganda neighbors the Democratic Republic of Congo (DRC) to the West, which had been experiencing an outbreak of mpox for more than a year before Uganda confirmed a case. Uganda is divided into 146 administrative units known as districts. It has a population of 45.9M people with a high HIV prevalence of about 1.2M PLHIV (3, 4).
We conducted a cross-sectional study of all mpox confirmed cases admitted in the isolation and treatment units during the study period. The study was conducted in Entebbe, Nakasongola, and Mbarara isolation and treatment units. We also collected data in health facilities in Mayuge and Masindi Districts. By the time of the study, the country was implementing isolation of all confirmed cases.
We included all cases confirmed to have had mpox using polymerase chain reaction (PCR) test for mpox.
To ascertain the degree of severity, we assessed all mpox cases identified using the adapted MPSSS tool. The MPSSS tool is a 7-parameter numerical scoring tool comprising number of lesions, number of body parts affected, confluency of the lesions, secondary bacterial infection, mucosal involvement, degree of pain and the optimal level of care for case-patients. Each parameter is scored on a scale of 0-4; the total score ranges from 0-23. We used a cutoff of ≥7 for severe disease. We also obtained information from the cases on their socio-demographics, comorbidities, and other clinical information such as pregnancy status for women from the case investigation forms (CIF).
We applied the MPSSS to obtain mpox severity scores. We described the cases and estimated the prevalence of mpox severity. Wilcoxon rank sum test was used to compare the severity scores among different groups of patients according to their background characteristics.
The Ministry of Health of Uganda gave the directive and gave approval to conduct this study. The Office of the Associate Director for Science at the US Centres for Disease Control and Prevention (CDC) Uganda determined that this research did not involve human subject research and that its primary intent was public health practice or disease control. Verbal informed consent was obtained from participants or, if the interviewee was a minor, guardians before the start of each interview.
Results
We applied the adapted MPSSS tool to 224 confirmed mpox cases for severity. The majority of the mpox cases presented with rash 100% (243/244), followed by fever 83% (203/244), local lymphadenopathy 67% (167/244), and sore throat 52% (126/244). Of the 244 cases, 30% (74/244) had comorbidities. Of the 74 cases who had underlying conditions, 88% (65/74) had HIV. The other underlying conditions included pregnancy, multidrug resistant tuberculosis (MDR TB), sickle cell disease, peptic ulcer disease, and hypertension.
Of the 244 cases, 52% (128/224) were males, the median age was 29 years, interquartile range (IQR)=24-38, range=1day-54years (Table 1).
Table 1: Baseline characteristics of mpox patients during the mpox outbreak, Uganda, 2024–2025 (n=244)
| Characteristic | Frequency (n) | Percentage (%) |
| Gender | ||
| Female | 116 | 48 |
| Male | 128 | 52 |
| Occupation | ||
| Waitress | 27 | 12 |
| Vendor | 24 | 11 |
| Business person | 23 | 10 |
| Sex Worker | 22 | 9.8 |
| Shop Keeper | 18 | 8.0 |
| Agriculture | 17 | 7.6 |
| Student | 15 | 6.7 |
| Builder | 10 | 4.5 |
| Driver | 10 | 4.5 |
| Salon attendant | 10 | 4.5 |
| Others | 48 | 20 |
| Age-group | ||
| 0-5 years | 8 | 3.3 |
| 5-17 years | 14 | 5.7 |
| 18-29 years | 104 | 43 |
| 30-41 years | 88 | 36 |
| 42-53 years | 29 | 12 |
| 54-65 years | 1 | 0.41 |
| Median (IQR | 29(24-38) | |
| Underlying condition | ||
| No | 170 | 64 |
| Yes | 74 | 36 |
| HIV status | ||
| Negative | 155 | 70 |
| Positive | 65 | 30 |
| Pregnancy status | ||
| No | 95 | 95 |
| Yes | 5 | 5 |
| Outcome | ||
| Alive | 192 | 93 |
| Deceased | 14 | 6.8 |
| Severity | ||
| Mild | 60 | 25 |
| Severe | 184 | 75 |
Severity of mpox
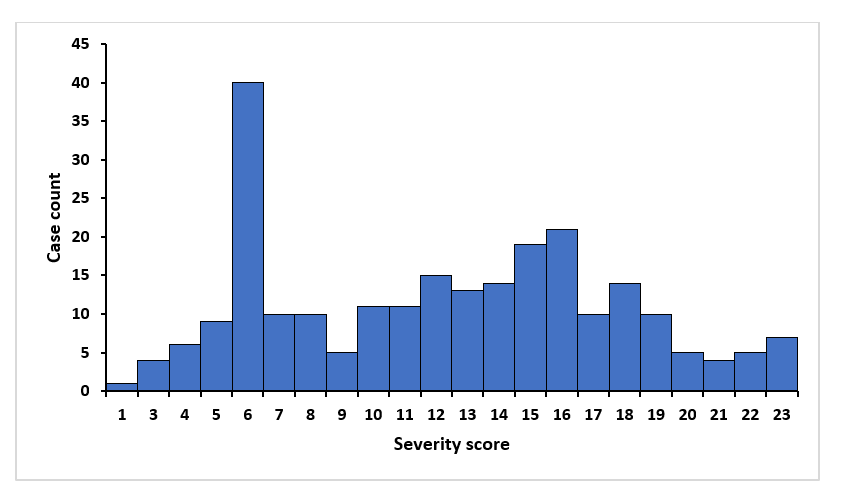
The majority of the cases were severe, 75% (184/224). The median severity score was 12.5 with a range of 1-23 with right-sided skewness (Figure 1). Out of the 224 cases, 6.8% of the cases died (14/224) (Table 1). Among these deaths, 71% (10/14) of them had comorbidities and 50% (7/14) scored 23/23 using the MPSSS tool. Of the 10 who had comorbidities, 9 had HIV infection and the other had sickle cell anemia. Among the deaths, most 43% (6/14) were aged 30-45years, followed by <5years of age who contributed 21% (3/14), 15-30 years of age contributed 14% (2/14), 45-60years of age contributed 14% (2/14), and 5-15 years of age contributed 1% (1/14).
Mpox severity score and background characteristics
We analysed the relationship between the background characteristics of the mpox patients and their severity scores using the Wilcoxon rank-sum test. We found no significant statistical difference when females where compared to males (p=0.72). We categorized age into children (0-17 years) and adults (≥ 18 years), and we found no statistical difference between the two groups but children were more likely to have higher severity scores (p=0.25). Mpox patients with any underlying illness and PLHIV were more likely to have higher severity scores when compared to those without (p<0.005). Pregnant women were more likely to have a higher severity score compared to non-pregnant women but with no statistical significance (p=0.58) (Table 2).
Table 2: Comparison of severity scores and the background characteristics of mpox cases, November 2024–February 2025, Uganda
| Characteristic | Frequency (n) | Rank-sum | Expected | p value |
| Age | ||||
| < 17 years | 22 | 2,332 | 2,695 | 0.25 |
| 18–55 years | 222 | 27,588 | 27,195 | |
| Gender | ||||
| Female | 116 | 15,481 | 15,680 | 0.72 |
| Male | 128 | 14,410 | 14,210 | |
| Pregnancy | 0.59 | |||
| Pregnant | 7 | 279 | 245 | |
| Non-pregnant | 93 | 4,475 | 4,508 | |
| Underlying illness | ||||
| Yes | 74 | 12,352 | 90,655 | <0.001 |
| No | 170 | 17,359 | 20,825 | |
| HIV status | ||||
| Positive | 65 | 10,159 | 7,183 | <0.001 |
| Negative | 155 | 14,151 | 17,128 | |
Discussion
Our findings contribute to further characterization of the current mpox pandemic, focusing on the severity of the disease. We found a high prevalence of severe mpox and a high proportion of deaths among the cases.
We defined a severe disease using a severity score cutoff ≥7 which demonstrated good predictive ability in settings with high HIV prevalence in a study that piloted the MPSSS tool (2). We recommend adopting the ≥7 cutoff in our setting and other similar settings given the high HIV prevalence. The high prevalence of severe mpox in this study was likely due to the high HIV prevalence in our setting. HIV has been found to be both a risk factor for mpox infection and also for severe mpox. Other studies have found similar findings, with PLHIV more likely to have severe presentations of mpox compared to those without HIV. Case-patients with HIV were also more likely to be hospitalized compared to those without HIV (5, 6).
In our study, the proportion of deaths among the cases was high, and living with HIV was a big contributor. These deaths also had high severity scores. Other studies have found a similar pattern of increased risk of death among PLHIV with mpox (5, 7). Prioritizing people with comorbidities for vaccination, especially PLHIV and other comorbidities, can protect them against severe disease. Cases with low severity scores and with no known comorbidities can be considered for home-based care and monitoring, whereas cases with higher scores would be prioritized for more inpatient care and close monitoring.
Strengths
To our knowledge, this was the first study to assess for severity of mpox in the country using the MPSSS tool. It has demonstrated consistency in the clinical presentation and outcome of mpox patients with their respective severity scores.
Study limitations
We applied the MPSSS tool at one point in time and only updated for the outcome. Some changes could have occurred as the mpox disease progressed. There could also have been an overestimation of the scores due to subjective assessment of secondary bacterial infection on clinical basis for most of the cases without carrying out a culture and sensitivity.
Conclusion
We found a high prevalence of mpox severity, with PHIV having an increased likelihood of severe disease. We recommend prioritizing case-patients with underlying conditions, especially PLHIV, for inpatient care and vaccination to protect them from severe mpox disease.
Conflict of interest
The authors declared no conflict of interest.
Authors’ contribution
All authors contributed to the write-up and review of the bulletin. EM drafted the initial version of thebulletin. RM, PE, BK, LB, and ARA revised the manuscript for substantial intellectual content. EM, JOK, EOO, DW, JLK, PK, JN, CM, HK, LON, AMN, BA, and GA participated in the data collection and case investigations. RM, LB, BK, IL, PE, and ARA supervised the field data collection and reviewed the draft bulletin for substantial intellectual content. All authors read and approved the final bulletin.
We acknowledge the support of the health workers and research assistants in the different isolation and treatment units who participated in assessment of the case-patients and the data collection. We also acknowledge the support of the Ministry of Health, Uganda for the logistical support in form of personnel protective equipment and the entire outbreak response.
Copyright and licensing
All materials in the Uganda Public Health Bulletin are in the public domain and may be used and reprinted without permission; citation as to source, however, is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- Clinical review of human mpox. Clinical Microbiology and Infection. 2023 Dec 1;29(12):1493–501.
- Zucker J, McLean J, Huang S, DeLaurentis C, Gunaratne S, Stoeckle K, et al. Development and Pilot of an Mpox Severity Scoring System. The Journal of Infectious Diseases. 2024 Apr 15;229(Supplement_2):S229–33.
- Uganda Bureau of Statistics. Uganda Bureau of Statistics 2024: The National Population and Housing Census 2024 – Preliminary Report, Kampala, Uganda [Internet]. 2024 Jul. Available from: https://www.ubos.org/
- Ministry of Health (Uganda). Uganda Population-based HIV Impact Assessement 2020-2021 (UPHIA 2020-2021) final report [Internet]. Kampala, MoH, Uganda; 2024 Apr. Available from: https://phia.icap.columbia.edu/
- Taha AM, Elrosasy A, Mahmoud AM, Saed SAA, Moawad WAET, Hamouda E, et al. The effect of HIV and mpox co-infection on clinical outcomes: Systematic review and meta-analysis. HIV Medicine. 2024;25(8):897–909.
- Silva MST, Coutinho C, Torres TS, Peixoto EM, Bastos MO, Mesquita MB, et al. Mpox severity and associated hospitalizations among people with HIV and related immunosuppression in Brazil. AIDS. 2024 Jan 1;38(1):105.
- Laurenson-Schafer H, Sklenovská N, Hoxha A, Kerr SM, Ndumbi P, Fitzner J, et al. Description of the first global outbreak of mpox: an analysis of global surveillance data. The Lancet Global Health. 2023 Jul 1;11(7):e1012–23.


Comments are closed.