Food poisoning outbreak caused by Aeromonas bacteria at a funeral in Buyengo Town Council, Jinja District, Uganda, February 2024
Authors: Yasiini Nuwamanya1*, Innocent Ssemanda1, Dorothy Aanyu1, Brian Kibwika1, Shem S. Mwebaza1, Yunus Mbwire2, Gorreti A. Olupot3, Peruth Bamukisa3, Joshua Kayiwa4, Hildah Tendo Nansikombi1, Benon Kwesiga1, Lilian Bulage1, Richard Migisha1, and Alex R. Ario1; Institutional affiliations: 1Uganda Public Health Fellowship Program, Uganda National Institute of Public Health, Kampala, Uganda, 2Jinja District Local Government, Uganda, 3Busoga Regional Emergency Operations Centre, Uganda, 4Public Health Emergency Operations Centre, Ministry of Health, Uganda; Correspondence*: Tel: +256773120564, Email: ynuwamanya@uniph.go.ug
Summary
Background: Aeromonas, gram-negative toxin-producing bacteria, are emerging human pathogens for food poisoning, causing acute gastroenteritis with an incubation period of 12 hours-7 days. On February 15, 2024, Ministry of Health was notified of suspected food poisoning following a funeral in Buyengo Town Council (TC), Jinja District where seventy-two people developed gastrointestinal symptoms. We investigated to determine the cause, magnitude and risk factors for the outbreak to inform control and prevention measures.
Methods: We defined a suspected case as onset of abdominal pain and ≥1 of the following: diarrhea, vomiting or nausea in a resident/ visitor of Buyengo TC in Jinja District and Nawampiti Subcounty (SC) in Luuka District during February 11–22, 2024. We found cases through health facility records review and community case search; collected data using an interviewer-administered questionnaire on identified cases.
We conducted descriptive epidemiology and environmental assessments to generate hypotheses. We conducted an unmatched case control study among funeral attendees in the two most affected villages, and microbiology and toxicology laboratory tests on 20 case-patients and 14 environmental samples.
Results: We identified 65 case-patients; 5% case-fatality rate. Common symptoms included abdominal pain (100%), diarrhea (94%) and vomiting (51%); 34% reported fever. Sixty-one (94%) case-patients had attended a funeral in Buyengo TC. The epidemic curve revealed multiple peaks, about 12 hours apart corresponding to the different serving times for the case-patients at supper and breakfast. Most cases presented within 12-86 hours from Monday supper time and 2-70 hours from Tuesday breakfast at the funeral; median incubation period was 34 hours (range=12-211 hours). For both meals, beef soup served was topped-up with unboiled water and not properly re-cooked. Sixty-two percent of the cases compared to 38% of the controls ate beef stew at supper (OR=2.7; 95%CI=1.2, 6.2). Additionally, 97% of the cases compared to 40% of the controls ate leftover beef stew for Tuesday breakfast (OR=57; 95%CI=5.4, 600). The main source of water used at the funeral was ‘Kabakubya’ stream. Aeromonas hydrophilia and Aeromonas caviae were isolated in the gastric aspirate from one of the case-patients, and the water from the stream.
Conclusion: This was a point source food poisoning outbreak caused by consuming beef stew contaminated with Aeromonas at a funeral. The Aeromonas was traced to a nearby stream. Stopping the use of water from the stream and enhanced water, sanitation and hygiene interventions helped control the outbreak.
Background
Food poisoning, a common public health problem that can cause severe illness and death, occurs when two or more people get a similar illness after consuming the same contaminated food or drink (1) . It’s estimated that food poisoning affects 600 million people, killing 420,000 every year globally (2). Food poisoning can be caused by various agents including biological, viral, natural toxin, and chemicals that can contaminate food during production, processing, distribution, or preparation (1, 3).
In Uganda, the most common food poisoning causes are bacteria such as Salmonella, Escherichia coli (E. coli), and Staphylococcus aureus (S. aureus), Shigella, Campylobacter spp, Bacillus cereus (B. cereus), and Clostridium species (spp) (4-6). The bacteria exist in their natural environment such as water and can contaminate meat, eggs, dairy products, food and vegetables and cause large food poisoning outbreaks during social functions (4, 5). Bacterial food poisoning can be infectious, toxic or mixed in nature. The toxins can be produced in the food before ingestion (pre-formed toxins) or in the gut after ingestion (enterotoxins). Toxin-producing bacteria such as E. coli, S. aureus, B. cereus, Clostridium, and Aeromonas usually cause acute symptoms in a few hours (2-24hrs) and can mimic chemical and natural toxins (3).
Aeromonas, gram-negative toxin-producing aquatic bacteria, are emerging human pathogens known to cause food poisoning including acute gastroenteritis and septicemia. Four species including Aeromonas caviae, Aeromonas dhakensis, Aeromonas veronii, and Aeromonas hydrophilia account for >96% of the incidents.
Food poisoning due to Aeromonas usually presents with abdominal pain or cramps, diarrhea which maybe bloody, vomiting, and nausea with or without fever; incubation period of 12 hours to 7 days (7-10). Some of the known risk factors for food poisoning include poor hygiene practices, inadequate cooking methods, improper food storage conditions, and lack of awareness among consumers.
On February 15, 2024, Ministry of Health was notified of a suspected food poisoning outbreak in the Buyengo Town Council (TC) in Jinja District. The alert followed several people from the town council, complaining of acute onset of abdominal pain and diarrhea. Many of the sick reported attending a funeral in Bukasami village, Buyengo TC in the preceding days. We investigated to determine the cause, magnitude and risk factors for the outbreak to inform control and prevention measures.
Methods
Outbreak area
The outbreak occurred in Buyengo TC in Jinja District, and the neighbouring Nawampiti SC in Luuka District respectively. The suspected funeral took place in Bukasami village, in Buyengo TC which borders Nawampiti SC to the north (Figure 1). The deceased was the head of household and area Muslim leader and the funeral lasted several days attracting a lot of attendees, mainly from Buyengo TC and Nawampiti SC. He was buried on Monday, February 12, 2024, one day after his death but a prayer ceremony was held on the following day. On both occasions, funeral attendees were served different foods and drinks at breakfast, lunch, and supper.
We defined a suspected case as onset of abdominal pain and ≥1 of the following: diarrhea, vomiting or nausea in a resident/ visitor of Buyengo Town Council (TC) in Jinja District and Nawampiti Subcounty (SC) in Luuka District during Feb 11–22, 2024. A confirmed case was a suspected case with laboratory confirmation of causative agent in a clinical specimen.
We conducted case finding using record reviews at the three health facilities serving the affected area including one health center III, one general hospital, and a regional referral hospital. We also conducted active case search within the affected communities. We interviewed cases using a case investigation form and developed a line list. The case investigation form included information on demographic characteristics, clinical features such as the time of onset and duration, social history, foods and drinks taken on specific days and their source, any laboratory investigations done, treatment given, and outcomes
We conducted descriptive analysis of data from interviews with case-patients. We characterized cases by person, place, and time and possible exposures. Person characteristics included sex and age. We categorized age (in years) into six meaningful categories including 0-4 (younger children), 5-14 (older children), 15-24 (young persons), 25-39 (young adults), 40-59 (middle aged) and ≥60 (elderly). We used the 2023 population estimates from the Uganda Bureau of Statistics (UBOS) for Jinja and Luuka districts to calculate attack rates by sex, age group, subcounty and village (11). We constructed chloropleth maps using the Quantum Geographic Information System (QGIS) software to display attack rates by place. We used an epidemic curve to analyse the distribution of cases by time of symptom onset.
Case management
A treatment unit was established at Buwenge General Hospital, the main hospital for Jinja District. The hospital is located in Buwenge Subcounty which borders Buyengo TC, the epicenter of the outbreak. Suspected cases were evacuated to the hospital from the communities by the district and Red Cross health teams using ambulances.
While at Buwenge General Hospital, the case-patients were admitted for observation and management. The case-patients were treated mainly with intravenous fluids to correct severe dehydration, and antibiotics including Ceftriaxone and Ciprofloxacin for suspected bacterial infection. Four of the case-patients were referred to Jinja Regional Referral Hospital for further management
We observed the funeral site and interviewed several key informants including two widows of the late AM and one of the cooks. We also interviewed the local council chairpersons of Bukasami and Nawandyo villages and household heads/caretakers of the deceased case-patients. Interviews focused on circumstances surrounding AM’s death, burial practices, source of water, foods prepared at the funeral, the food preparation and handling processes. We also searched for any dumped leftover food at the funeral site. We collected water samples from the different sources of water used at the funeral, including four boreholes and ‘Kabakubya” stream in Bukasami village.
Laboratory investigations
We collected both clinical and non-clinical samples from case-patients and the environment for laboratory testing. The clinical samples included one gastric aspirate, eight stool samples, 74 blood samples, and four urine samples from 53 case-patients. The non-clinical samples included four leftover food samples, piece of a soiled mattress used by one of the case-patients, and ten water samples. The water was collected from five water sources said to have been used during the funeral, and one jerrycan from the household where the funeral was held. The clinical and non-clinical samples including water samples were sent to Uganda’s Central Public Health Laboratories (CPHL) for microbiology. Additionally, some clinical samples and the non-clinical samples such as leftover food were sent to the Directorate of Government Analytical Laboratory (DGAL) for toxicology screening.
We conducted 65 interviews among case-patients that were identified through active case-search, including all the deceased. We also interviewed members of the district health team that were among the first responders, clinicians that attended to the case-patients, and the medical superintendent of Buwenge General Hospital where most of the case-patients were managed. We obtained data on social functions attended, the circumstances surrounding AM’s death, funeral activities, foods and drinks taken prior to symptom onset, specific foods and drinks served at the funeral including the source, source of water used for cooking and hand washing, food preparation and handling during the funeral, clinical presentation of cases, and case management.
We used the results of the descriptive analysis of case-patient data, key informant interviews, and environmental findings to generate hypotheses.
Case Control Study
To test the hypotheses, we conducted an unmatched case-control study among the funeral attendees in the two most affected villages of Bukasami and Nawandyo in Buyengo TC and Nawampiti SC respectively. For each case-patient, we selected 3 control persons. A control was a resident or visitor to Bukasami and Nawandyo villages in Buyengo TC and Nawampiti SC, who attended the suspected funeral but had no history of abdominal pain, diarrhea or vomiting during February 11–22, 2024.
We used village house-hold lists to generate sampling frames per village and selected one control per household from the list of non-case households using simple random sampling. In total, 61 cases and 183 controls were selected. An interviewer administered questionnaire was administered to the eligible case-patients and control-persons to obtain information on their demographic, clinical characteristics, and foods and drinks taken at the funeral among others. We identified risk factors associated with the outbreak using logistic regression.
This investigation was conducted in response to public health emergency by the National Rapid Response Team. The Ministry of Health Uganda provided administrative clearance to conduct this investigation. In addition, we received a non-research determination clearance from the US Centers for Disease Prevention and Control (US CDC). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy. § §See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq. Furthermore, all the respondents gave individual verbal consent or assent for interviews since the investigation presented no more than minimal risk of harm and involved no procedures for which written consent is normally required in other contexts. We conducted the interviews in privacy to ensure confidentiality and the data kept under password protection by the study team.
Results
Descriptive epidemiology
Person and clinical characteristics of case-patients in a food poisoning outbreak caused by Aeromonas bacteria, Jinja and Luuka districts, Uganda,February 2024 (n=65)
Overall, 65 case-patients were identified including one confirmed case; 5% (3/65) case-fatality rate. The confirmed case-patient had only taken leftover fried rice pre-mixed with leftover beef stew from the supper that was served on Monday, 12th February 2024. The leftover fried was carried home by the father from the funeral on Tuesday, February 12th 2024. The majority (52%) of case-patients were female. The median age was 20 years (IQR = 9-36) and most were aged 5-14 years (Table 1).
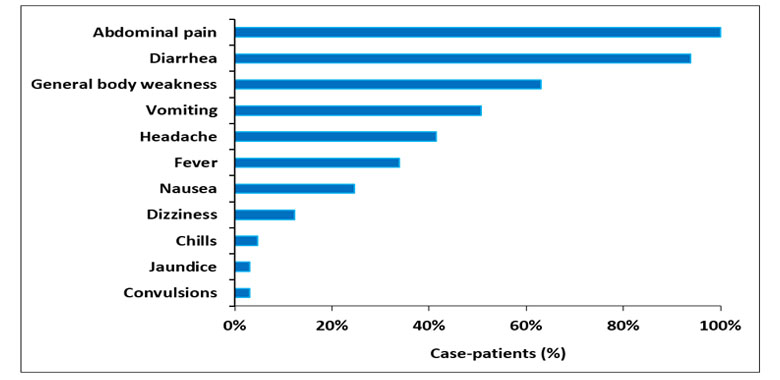
In addition to severe abdominal pain/cramps, the majority (94%) of the case-patients reported diarrhea and vomiting (51%). Fever was reported in only 34% of the case-patients. Among the non-specific symptoms, general body weakness was the commonest (63%) followed by headache at 42% (Figure 2). In 92% (60/65) of the case-patients, the first symptom was severe abdominal pain/cramps, with only 5% experiencing diarrhea as the first symptom. Among those with diarrhea, 25% had bloody diarrhea. Overall, females (AR=11/10,000) and males (AR = 10/10,000) were similarly affected. The elderly (≥60 years) (AR=27/10,000) were the most affected, while the 0-4 year age-group was least affected (Table 1).
Table 1: Attack rates by sex and age group during a food poisoning outbreak caused by Aeromonas bacteria, Jinja and Luuka districts, Uganda, February 2024
Distribution of case-patients by time of symptom onset during the food poisoning outbreak caused by Aeromonas bacteria, Jinja and Luuka districts, Uganda, February 2024
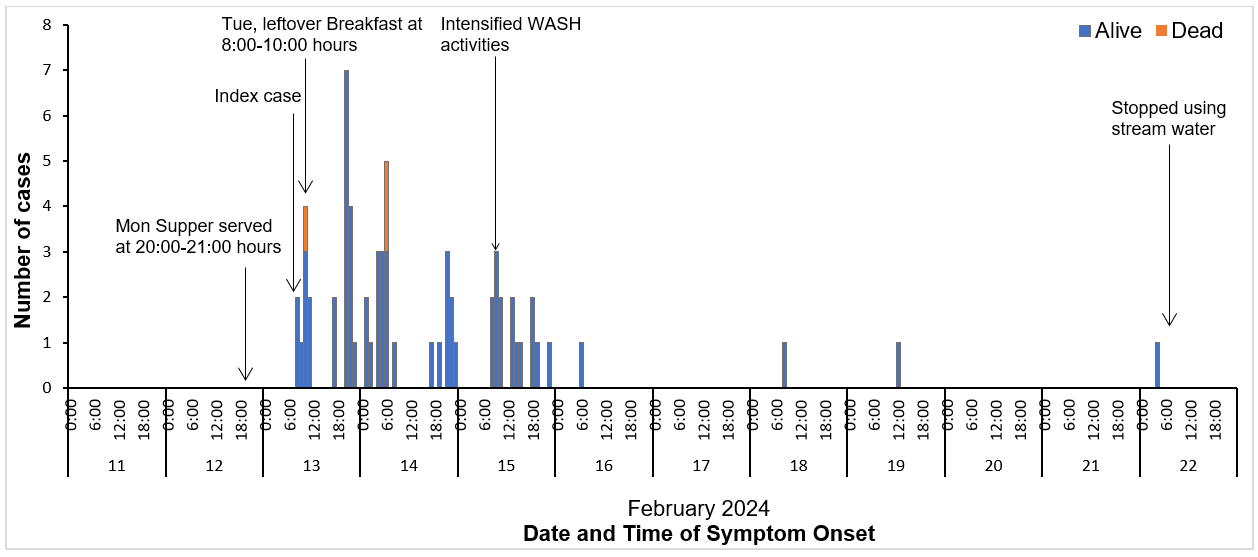
The majority (94%) of the case-patients reported to have attended the funeral of AM, a prominent man in Jinja District who died on Sunday February 11, 2024. Funeral attendees started arriving on the same day, but no meals were served on that day. The following day on Monday February 12, 2024, at around 10:00 hours, funeral attendees were served breakfast with black tea and no accompanying food item. Later in the day, from 13:00-14:00 hours, the funeral attendees were served lunch with foods including: brown (fried) rice, white (boiled/unfried) rice, goat’s meat and beef stew. Later on the same day, supper comprising brown rice and beef stew was prepared separately and served from 20:00-21:00 hours. However, one sauce pan containing brown rice that had been prepared for supper along with another containing leftover beef stew were kept and served to the funeral attendees at breakfast the following day, Tuesday February 13, 2024, between 8:00-10:00 hours. For both Monday supper and Tuesday breakfast, the beef stew was topped up with water to meet the high demand and was not properly re-cooked. We found that 94% (61/65) of the case-patients had attended the funeral, and 90% (55/61) had eaten either supper on Monday or breakfast served the following day using leftover food and beef stew from the previous supper (Table 2).
Case-patients started experiencing symptoms on Tuesday February 13 2024 at 08:00hrs. The epicurve revealed multiple peaks with a time interval of about 12-24 hours between; suggesting a point source outbreak with multiple exposure times corresponding to the different serving times for the case-patients at supper and breakfast. Additionally, most of these cases presented within a time interval of 12-86 hours from when Monday supper was served and 2-70 hours from when breakfast was served the following day on Tuesday. The estimated median incubation period for case-patients was 37 hours (range = 12-185 hours) from their respective serving times for Monday supper and 27 hours (range = 0-211 hours) from their serving times for Tuesday breakfast. We noted that seven (11%) of the case-patients developed symptoms before or around the serving time for Tuesday breakfast but had taken supper at least 12 hours prior, suggesting that they had been exposed at supper time (Figure 2). In addition, all case-patients whose estimated incubation periods were less than12 hours from their breakfast serving time on Tuesday had also taken supper the previous day. Considering Monday supper as the earliest point of potential exposure, the overall median incubation period was estimated at 34 hours (range 12-211 hours) (Figure 2).
Attack rates by village and subcounty during a food poisoning outbreak caused by Aeromonas bacteria, Jinja and Luuka districts, Uganda, February 2024
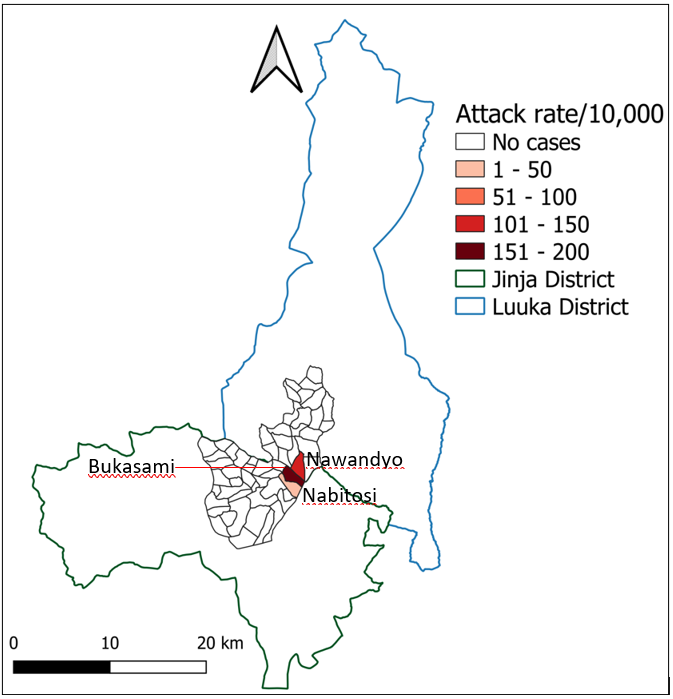
Buyengo TC in Jinja District (AR = 11/10,000) and Nawampiti Subcounty (AR = 10/10,000) were similarly affected. However, Bukasami Village in Buyengo TC where the funeral was held was the most affected village (159/10,000) (Figure 3).
Environmental findings
We found that whereas the main source of water routinely used by residents was the boreholes, most of the water used at the funeral was fetched from ‘Kabakubya’ stream using boda bodas and a pick-up motor vehicle. This was reported to have been necessitated by urgency and increased demand for water because the funeral attracted >1000 funeral attendees. We also found that unboiled water from ‘Kabakubya’ stream that was added to top-up the beef soup at Monday supper and the beef stew was not allowed enough time to be re-cooked. The beef stew was served with freshly prepared brown rice at supper on Monday and leftover brown rice from the same supper at breakfast the following day. It was reported that all the leftover brown rice served for breakfast was already pre-mixed with the beef stew.
Laboratory investigation findings
Aeromonas hydrophilia and Aeromonas caviae were isolated from the gastric aspirate of the deceased 4-year-old case-patient, revealing mixed infection by different strains of Aeromonas bacteria. Similarly, the water sample from Kabakubya water stream tested positive results for Aeromonas hydrophilia by culture. The leftover food samples tested negative on bacteriological culture and toxicology.
Hypothesis generation findings
We found that 61(94%) of the case-patients had attended AM’s funeral on either Monday, February 12, 2024 or Tuesday, February 13, 2024 or both, and all had taken at least one meal at the funeral. Most (90%) of the case-patients had taken either Monday supper or Tuesday breakfast served with beef stew. The beef stew had been prepared at supper on Monday, February 12, 2024 but the leftovers were served at breakfast the following day (Table 2). It was reported that during both meals, the beef stew was topped up using water from a local stream to meet the demand from the funeral attendees, and was never properly recooked.
We thus hypothesized that the food that was served at either supper on Monday, February 12 or breakfast on Tuesday, February 13, 2024 was contaminated by an infectious causative agent. The agent was likely in the unboiled stream water that was used to top-up beef stew that was not properly re-cooked, and served at supper on Monday and as leftover breakfast the following day.
Table 2: Potential exposures associated with food poisoning caused by Aeromonas bacteria, Jinja and Luuka districts, Uganda, February 2024
| Potential exposure | Cases | Percentage |
| Attended AM’s funeral (N=65) | 61 | 94 |
| Attended the funeral on Mon (N=61) | 52 | 80 |
| Attended on Tue-13 (N=61) | 49 | 75 |
| Ate any food at the funeral (N=61) | 61 | 100 |
| Ate any food onTue-13 (N=61) | 47 | 77 |
| Ate breakfast on Tue (N=47) | 38 | 81 |
| Ate Lunch on Tuesday (N=47) | 25 | 53 |
| Ate Supper on Tuesday (N=47) | 23 | 49 |
| Ate any food on Mon-12 (N=61) | 44 | 72 |
| Ate supper on Mon (N=44) | 43 | 98 |
| Ate Lunch on Monday (N=44) | 21 | 48 |
| Ate either Mon supper or Tue breakfast (N=61) | 55 | 90 |
| Took any drink at the funeral (N=61) | 26 | 42 |
| Attended the funeral on Wed (N=61) | 14 | 23 |
Risk factors for food poisoning caused by Aeromonas bacteria among case-patients, Buyengo TC, Jinja District and Nawampiti SC, Luuka District, Uganda, February 2024
Eighty-six percent of the cases compared to 43% of the controls ate food at the funeral on Monday (cOR=7.2; 95% CI=3.2, 16.3). Similarly, 96% of the cases compared to 50% of the controls ate food at the funeral on Tuesday (cOR=24; 95% CI = 4.9, 112.6). Among those who ate food on Monday, 62% of cases compared to 38% of the controls ate beef at supper (aOR=2.7; 95% CI=1.2, 6.2). Furthermore, 97% of the case-patients compared to 40% of the control-persons ate leftover beef stew for Tuesday breakfast (cOR=57, 95% CI=5.4, 600).
An equivalent proportion of case-patients (97%) compared to control-persons (40%) ate leftover brown rice pre-mixed with the leftover beef stew for Tuesday breakfast (cOR=57, 95% CI=5.4, 600). However, eating brown rice at supper was not significantly associated with food poisoning (aOR=1.2, 95% CI=0.1, 11). Similarly, common reference group analysis also revealed that eating the brown rice without beef stew for Monday supper was not associated with food poisoning (aOR=2.6; 0.50,13) (Table 3). Dose-response analysis in the relationship between beef stew and food poisoning revealed no linear trend.
Table 3: Risk factors for food poisoning caused by Aeromonas bacteria among case-patients, Buyengo TC, Jinja District and Nawampiti SC, Luuka District, Uganda, February 2024
| Exposure | Cases (%) | Controls (%) | cOR (95% CI) | aOR (95% CI) |
| Ate any food at the funeral on Monday | ||||
| Yes | 44(85) | 65(43) | 7.2 (3.2,16) | |
| No | 8(15) | 85(57) | Ref | |
| Ate any food at the funeral on Tuesday | ||||
| Yes | 47(96) | 17(50) | 24 (4.9,112) | |
| No | 2(4) | 17(50) | Ref | |
| Ate beef stew for Mon supper | ||||
| Yes | 26(62) | 23(38) | 2.6 (1.2,5.9) | 2.7(1.2,6.2) |
| No | 16(38) | 37(62) | Ref | |
| Ate brown rice for supper on Monday | ||||
| Yes | 36(86) | 44(73) | 2.2 (0.8,6.2) | 1.2 (0.1,11) |
| No | 6(14) | 16(27) | Ref | |
| Ate brown rice with beef stew for supper on Monday | ||||
| Yes | 22(54) | 19(33) | 2.4 (1.02,5.5) | |
| No | 17(33) | 35(67) | Ref | |
| Ate goat meat for supper on Monday | ||||
| Yes | 1(2) | 1(2) | 1.4 (0.1,24) | |
| No | 41(98) | 59(98) | Ref | |
| Ate white rice for supper on Monday | ||||
| Yes | 6(14) | 16(27) | 0.5 (0.2,1.3) | 0.5 (0.052, 4.7) |
| No | 36(86) | 44(73) | Ref | |
| Ate leftover beef stew for breakfast on Tue | ||||
| Yes | 38(97) | 4(40) | 57 (5.4,600) | |
| No | 1(3) | 6(60) | Ref | |
| Ate leftover brown rice for breakfast on Tue (all served with leftover beef stew) | ||||
| Yes | 38(97) | 4(40) | 57 (5.4,600) | |
| No | 1(3) | 6(60) | Ref | |
| Took black tea at breakfast on Tuesday | ||||
| Yes | 11(69) | 13(59) | 1.5 (0.39,5.9) | |
| No | 5(31) | 9(41) | Ref | |
#cOR refers to crude Odds Ratio
*aOR refers to adjusted Odds Ratio, *Beef stew and brown rice pre-mixed beef stew eaten on Tuesday were not included in the multivariate models despite having a significant association with food poisoning at bivariate analysis, due to collinearity.
Discussion
Our investigation revealed a point source outbreak of food poisoning caused by Aeromonas bacteria in Buyengo TC in Jinja District and Nawampiti SC in Luuka District. Cases presented with symptoms of acute gastroenteritis, mostly severe abdominal pain/cramps and diarrhea, some of which was bloody. The overall case-fatality rate was 5% and the elderly people were the most affected age-group. The outbreak affected three neighboring villages and followed a funeral ceremony that was held for several days at Bukasami Village in Buyengo TC. Bukasami village, where the funeral was held was the most affected village.
We found that all case-patients had eaten at least one meal at the funeral either on the day of burial, Monday February 12, 2024 or the day after. Eating beef stew at Monday supper and/or leftover beef stew at breakfast on the following day was a risk factor for food poisoning. We found that the beef stew was contaminated with Aeromonas from the unboiled water that was added top up the soup without proper re-cooking. We traced the source of the Aeromonas to ‘Kabakubya’ stream from which most of the water used at the funeral was fetched.
Aeromonas are gram-negative, non-spore forming rods that live in aquatic environments worldwide (9, 10). Nineteen of the thirty-six known species are considered emerging pathogens to humans (8). Several studies have implicated Aeromonas species in causing food poisoning, wound infection and septicemia in several studies (7-10, 12). More than 96% of the incidents were caused by one of four species including Aeromonas caviae, Aeromonas dhakensis, Aeromonas veronii, and Aeromonas hydrophilia (8, 10, 13).
In this outbreak, the case-patients mainly experienced severe abdominal pain/cramps, and diarrhea in addition to vomiting. This symptom profile is consistent with the clinical features of acute gastroenteritis caused by Aeromonas bacteria. The bacteria are known to cause acute gastroenteritis with features including abdominal pain, diarrhea which maybe bloody or not, vomiting, nausea, and occasionally jaundice and dyspnea. The bacteria also produce enterotoxins and can cause severe symptoms in a short time including sepsis and death (8).
The bacteria have a low infective dose following natural exposure with a median dose of only 0.9 colony forming units (cfu) required for 1% illness risk (9, 10, 14). This infectious dose following natural exposure is thousand times lower than what has been estimated from challenge studies (15) . Studies have also found that the infectious dose for Aeromonas in diarrheal illnesses is comparable to that of well-known enteropathogenic bacteria such as Campylobacter and Salmonella species (8, 15). The gastric aspirate from the confirmed case-patient in this outbreak grew 3 cfu grown on culture, which was over three times the above median dose.
The estimated median incubation period of 34 hours and the range of 12-185 hours for 98% of the cases was consistent with the known incubation period for Aeromonas which ranges from 12 hours to 7 days (16, 17). Furthermore, most of the cases presented within two days from the potential exposure which is consistent with findings of a previous study in Bhutan in which 70% of the cases developed symptoms within 2 days of consuming beef stew from a carcass, contaminated with Aeromonas (12).
Contaminated water sources are an important source of Aeromonas (18). The most frequent route of entry of Aeromonas into humans is oral–fecal route, but the bacteria can also enter the body through wounds (9, 10, 14). Consumption of contaminated water and food are considered the main routes of transmission (19, 20). In this outbreak, case-patients consumed beef stew prepared using water from a stream which was subsequently found to contain Aeromonas hydrophilia. This was consistent with the known oral-fecal transmission of Aeromonas (20). Similarly, a food borne diarrheal disease outbreak investigation in a college in China found that students were exposed from consuming cucumber salads that had been rinsed with water contaminated with Aeromonas (21).
In this particular outbreak, we found mixed infection with Aeromonas hydrophilia and Aeromonas caviae in the gastric aspirate from the only confirmed case-patient. This particular case-patient was also among the three that died. Studies have shown that mixed infections by different Aeromonas species can occur and usually result into more severe disease as compared to when the infection is caused by a single species (8, 15). Whereas Aeromonas bacteria are known to infect both immunocompromised and immunocompetent people, the former are usually more affected. This explains why the attack rate was highest in the elderly.
The last case in this outbreak presented 10 days from the last potential point of exposure, beyond the typical incubation period of Aeromonas. However, studies have also found evidence of bacterial shedding by symptomatic and asymptomatic persons leading secondary transmission (8).
Study limitations
Ongoing criminal investigations into the incident interfered with the investigation. Subsequently, we were unable to reach many of the cooks as they denied participating in food preparation due to fear of legal consequences. However, we were able to interview the main cook involved in preparing the implicated meals.
The investigation started four days later after the incident had occurred, and several visiting funeral attendees had already left the outbreak area and couldn’t be reached for interviews. This might have underestimated the magnitude of the outbreak.
No food samples were collected from the suspected food particularly the beef stew. The only food samples sent for testing were found dumped at the funeral site one week after the incident, and weren’t useful for microbiology. Nevertheless, they were still useful for toxicological investigations which were negative.
Conclusion
This was a point source outbreak of food poisoning in Buyengo TC and Nawampiti SC in Jinja and Luuka districts respectively caused by consuming beef stew that was contaminated with Aeromonas at a funeral. The source of the Aeromonas was the unboiled water from ‘Kabakubya’ stream that was added to top-up the beef soup at Monday supper and Tuesday breakfast. We recommended stopping the use of water from ‘Kabakubya’ stream and enhancement of WASH interventions to prevent secondary transmission and mitigate the risk of future outbreaks.
Public health actions
We disseminated the findings to Jinja District Task Force (DTF), and leaders of the affected communities. Subsequently, the DTF resolved to enhance Water and Sanitation, and Hygiene (WASH) interventions in the affected villages including distribution of chlorine water treatment tablets to all households and replenishing chlorine dispensers at all the water collection points.
Recommendations
We recommended the following measures to control the outbreak and mitigate the risk of future foodborne disease outbreaks.
Stopping the use of water from ‘Kabakubya” stream indefinitely, since it was untenable to treat the stream water at source, and yet the village had several alternative safe water sources such as boreholes and pumped water tap systems.
Conducting community sensitization on causes and prevention of food poisoning with emphasis on personal hygiene, sanitation, food preparation and handling practices to prevent secondary cases and the risk of future foodborne disease outbreaks.
Intensifying and sustaining WASH interventions in the affected villages such as provision of chlorine for water treatment, encouraging people to boil drinking water and ensuring proper disposal of feces to halt further transmission of Aeromonas.
We also recommended refresher training of the district and health facility surveillance teams on food poisoning outbreak investigation. This would facilitate timely detection and proper response in any future outbreaks of a similar nature.
We also recommended orientation of the clinical teams on proper investigation, and management of food poisoning to avoid missed opportunities for identifying the causative agents in case of future outbreaks.
Conflict of interests
The authors declare that they have no conflict of interests.
Authors’ contributions
YN, IS, DA, BK, SSM, YM, GO, PB, and THN, designed the study and contributed to the data collection and analysis. YN led the writing of the manuscript. YN, BK, RM, LB, and ARA participated in bulletin writing and review to ensure scientific integrity and intellectual content. All the authors contributed to the final draft of the bulletin.
Acknowledgement
We appreciate the management of Jinja District Local Government and Jinja Regional Referral Hospital for the stewardship, and the community for their participation in this investigation. We also thank the Ministry of Health, Public Health Emergency Operations Centre for their technical support and the US CDC for funding this investigation.
Copyright and licensing
All materials in the Uganda Public Health Bulletin are in the public domain and may be used and reprinted without permission. However, citation as to source is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- Dewey-Mattia D. Surveillance for foodborne disease outbreaks—United States, 2009–2015. MMWR Surveillance Summaries. 2018;67.
- Bhaskar S. Foodborne diseases—disease burden. Food safety in the 21st century: Elsevier; 2017. p. 1-10.
- Aljamali NM, Najim M, Alabbasy A. Review on food poisoning (types, causes, symptoms, diagnosis, treatment). Global Academic Journal of Pharmacy and Drug Research. 2021;3(4):54-61.
- Bagumire A, Karumuna R. Bacterial contamination of ready-to-eat meats vended in highway markets in Uganda. African Journal of Food Science. 2017;11(6):160-70.
- Mugampoza D, Byarugaba G, Nyonyintono A, Nakitto P. Occurrence of Escherichia coli and Salmonella spp. in street-vended foods and general hygienic and trading practices in Nakawa Division, Uganda. American Journal of Food and Nutrition. 2013;3(3):167-75.
- Namara BG. Food poisoning caused by Bacillus cereus at a secondary school in Mukono District, Uganda 2023 [cited 2023]. Available from: https://uniph.go.ug/food-poisoning-outbreak-caused-by-bacillus-cereus-at-a-secondary-school-in-mukono-district-uganda-july-2023/.
- Miyagi K, Hirai I, Sano K. Distribution of Aeromonas species in environmental water used in daily life in Okinawa Prefecture, Japan. Environmental health and preventive medicine. 2016;21:287-94.
- Fernández-Bravo A, Figueras MJ. An update on the genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms. 2020;8(1):129.
- Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clinical microbiology reviews. 2010;23(1):35-73.
- Figueras MJ, Beaz-Hidalgo R. Aeromonas infections in humans. Aeromonas. 2015:65-108.
- Statistics UBo. Population & Censuses 2023. Available from: https://www.ubos.org/explore-statistics/20/.
- Tsheten T, Tshering D, Gyem K, Dorji S, Wangchuk S, Tenzin T, et al. An outbreak of Aeromonas hydrophila food poisoning in deptsang village, Samdrup Jongkhar, Bhutan, 2016. Journal of Research in Health Sciences. 2016;16(4):224.
- Latif Eugenín FL. Aeromonas, un microorganismo ambiental de importancia en salud humana y animal. 2015.
- Figueras M, Suarez-Franquet A, Chacon M, Soler L, Navarro M, Alejandre C, et al. First record of the rare species Aeromonas culicicola from a drinking water supply. Applied and Environmental Microbiology. 2005;71(1):538-41.
- Teunis P, Figueras MJ. Reassessment of the enteropathogenicity of mesophilic Aeromonas species. Frontiers in microbiology. 2016;7:216438.
- J. Smith GU. Aeromonas hydrophila 2009. Available from: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/aeromonas-hydrophila.
- Environmental Services Food HST. Aeromonas Fact Sheet 2023. Available from: https://www.hastings.gov.uk/content/env_health/pdfs/poisoning/Aeromonas_Fact_Sheet.pdf.
- Carusi J, Kabuki DY, de Seixas Pereira PM, Cabral L. Aeromonas spp. in drinking water and food: Occurrence, virulence potential and antimicrobial resistance. Food Research International. 2023:113710.
- Lin Z, Lu J, Wu S, Lin X, Zheng L, Lou Y, et al. A novel detection method for the pathogenic Aeromonas hydrophila expressing aerA gene and/or hlyA gene based on dualplex RAA and CRISPR/Cas12a. Frontiers in microbiology. 2022;13:973996.
- Gómez-Gómez B, Volkow-Fernández P, Cornejo-Juárez P. Bloodstream Infections Caused by Waterborne Bacteria. Current Treatment Options in Infectious Diseases. 2020;12:332-48.
- Zhang Q, Shi G-Q, Tang G-P, Zou Z-T, Yao G-H, Zeng G. A foodborne outbreak of Aeromonas hydrophila in a college, Xingyi City, Guizhou, China, 2012. Western Pacific surveillance and response journal: WPSAR. 2012;3(4):39.


Comments are closed.