Rapid Antiretroviral Therapy Initiation following roll out of Point-of-Care Early Infant Diagnosis Testing, Uganda, 2018- 2021
Authors: Stella M. Migamba1*, Nsubuga Edirisa Juniour1, Andrew Kwiringira1, Benon Kwesiga1, Steven N. Kabwama1, Mary Nakafeero2, Lilian Bulage1, Alex R. Ario1; Affiliations 1Uganda Public Health Fellowship Program, Uganda National Institute of Public Health, Kampala, Uganda, 2Makerere University School of Public Health, Kampala, Uganda; Correspondence: Email: smigamba@musph.ac.ug, Tel: +256 774662488
Summary Background: Uganda Ministry of Health recommends a first HIV DNA-PCR test at 4-6 weeks for early infant diagnosis (EID) of HIV-exposed infants (HEIs), immediate results return and initiation of antiretroviral therapy (ART) for HIV-positive infants. In 2019, MOH introduced point-of-care (POC) whole-blood EID testing in 33 health facilities and scaled up to 133 in 2020. We assessed turnaround time for test results and ART linkage before and after implementation of POC testing. Methods: We evaluated EID register data for HEIs at 10 health facilities with POC and high-volume EID testing minimum EID testing volume of 12 infants per month from 2018-2021. At each facility, we abstracted data for 12 months before and after POC rollout. We compared time to sample collection, results receipt, and ART initiation between periods using medians, Wilcoxon rank-sum, and log-rank tests. Results: Data for 4,004 HEIs were extracted, including 1,688 (42%) pre-POC and 2,316 (58%) during POC. Overall, ninety-four percent of infants (3,762/4,004) had a first EID test. Median age at sample collection was 44 (IQR 38-52) days pre-POC and 42 (IQR 38-52) days during POC (p<0.001). Overall 3,762 HEIs tested, 3,667 (97%) had test results. HIV-positive infants’ (n=69) median age at sample collection was 92 (IQR 45-120) days pre-POC and 127 (IQR 74-206) days during POC (p=0.03). HIV positivity rate was 1.7% (27/1,610) pre-POC and 2.0% (42/2,057) during POC (p=0.09). For all infants, median days from sample collection to results receipt by infants’ caregivers were 29 (IQR 16-54) pre-POC and 1 (IQR 0-28) during POC (p<0.001); among HIV-positive infants, median days were 22 (IQR 4-30) pre-POC and 0 (0-3) during POC (p<0.001). Pre-POC, 0% (0/23) HIV-positive infants started ART on the sample collection day compared to 40% (17/42) during POC; ART linkage by 60 days after sample collection was 91% (21/23) pre-POC and 100% (42/42) during POC (p<0.001). Conclusion: POC testing improved EID results turnaround time and ART initiation for HIV positive infants. Later age at testing among infants who turn HIV-positive suggests missed opportunities in identifying HIV-exposed infants.While POC expansion could further improve ART linkage and loss to follow-up, there’s need to examine barriers surrounding the POC target of initiating ART on the sample collection day.
Background
Global estimates in 2020 showed that Early Infant Diagnosis (EID) of HIV coverage is still low. Only 68% of HIV- exposed infants (HEIs) were tested within 2 months of age in 2020 (1). Despite this indicator, countries continue to scale up interventions to eliminate mother to child transmission (MTCT) of HIV. Uganda is one of the countries on track towards eMTCT and is being considered for World Health Organization (WHO) certification of pre-elimination status (2).
The standard of care for EID of HIV involves collecting dried blood spots (DBS) from HEIs at health facilities and conducting Polymerase Chain Reaction (PCR) tests on these samples at a specialized reference laboratory. The Uganda Ministry of Health (MOH) standard on EID testing, adopted from the WHO 2015 guidelines, recommends that infants born to HIV positive mothers should have their first PCR test done at 4-6 weeks of age or as soon as the infant is identified thereafter as being born to an HIV-positive mother (3). Also, infants aged <18 months suspected to have HIV or with unknown exposure status should be screened for exposure, tested if exposed, and immediately initiated on anti-retroviral therapy (ART) if HIV-positive (4). This initiation is known as linkage to ART. Providing rapid results decreases loss to follow-up and reduces mortality in HIV-infected children (4).
Although 69,207 (70.8%) HEIs in Uganda were tested for HIV before 18 months of age; only 44% of these got their 1st PCR test within 6 weeks of age between July 2017 and June 2018(2). To facilitate rapid turnaround time for HIV results in infants, in 2019 the Uganda Ministry of Health rolled out point-of-care (POC) EID testing in 33 health facilities across the country and scaled it up to 133 health facilities in 2020. POC testing is a convenient medical testing at the site of client PMTCT & HEI/EID service delivery to increase chances that the mother-infant pairs and clinical team will promptly receive results, thus enabling timely clinical decisions (5). The WHO recommends that virological testing results should be returned to the clinic or care giver as soon as possible and no later than 4 weeks after sample collection to facilitate early initiation of ART (6) and that HEIs should also be started on ART immediately they test positive for HIV (7).
Studies in Malawi and Mozambique showed that > 98% of infants that received POC testing got their results and >70% of them were started on ART on the same day they received their results (8, 9). The impact of point-of-care testing on EID turnaround time and linkage to ART among HIV-positive infants in Uganda is unknown. We assessed turnaround time and ART linkage before and after implementation of POC testing.
Methods
Study setting
We conducted the study for the period of February 2018- September 2021 at ten health facilities with POC EID testing. These included three regional referral hospitals (RRH) (Fort Portal RRH, Mubende RRH, and Kawempe RRH), four general hospitals (Kiboga Hospital, Lyantonde Hospital, Mityana Hospital, and Kyenjojo Hospital), and 3 health centre IVs (Kyegegwa HC IV, Mpigi HC IV, and Ssembabule HC IV). The health facilities were considered because they reported the highest numbers of HIV exposed infants (HEIs) tested for HIV in 2020, and had a minimum EID testing volume of 12 infants per month according to the District Health Information System version 2 (DHIS 2), the national online health database.
Study design and data source
We conducted a retrospective evaluation of data for HIV exposed infants at the ten health facilities before and after the implementation of POC EID testing. At each facility, we abstracted data from EID registers for 12 months following the roll out of POC testing at the facility. For comparison, we also abstracted data for 12 months before POC roll out (pre-POC period) when centralized testing at a reference laboratory was the standard of care. According to national guidelines, three DNA PCR tests are conducted for HIV exposed infants. The study utilized results for the first DNA PCR test.
Study variables and data collection
We used a questionnaire programmed into Kobo collect on tablet computers to collect demographic information including infant’s identification number, sex, date of birth, date of registration at the facility, date of collection and dispatch of first PCR test, dates results received, dates results given to care giver, ART enrollment status and date, and final EID outcome (discharged negative, referred for ART, lost, died negative, died positive). We used the date of registration of the infant to determine whether they were in the pre-POC period or in the POC period. DNA PCR results turnaround times were defined as the number of days from sample collection to return of results to the clinic and to the caregiver. For turnaround time from sample collection to results receipt at clinic, we used dates of results received at the clinic or date of last clinic visit for censored observations. For turnaround time from sample collection to results receipt by caregiver, we used dates of caregiver results receipt.
A sub-group analysis was conducted among infants that tested positive for HIV to assess turnaround times and the effect of POC testing on time to ART initiation. The primary outcome in this study was time to ART initiation of HIV positive infants. Time to ART initiation was defined as the number of days between dates of sample collection and initiation on ART whereas same day ART initiation was starting ART on the same day of sample collection and results receipt. Positivity rate was defined as the proportion of infants that tested HIV positive out of the number tested and had valid results. The secondary study outcome was time to first DNA PCR sample collection from birth. Only HEIs who had date of result returned to caregiver were included in the analysis for proportion of those with HIV test results or were considered to have received an HIV test result.
Data management
Trained research assistants working in the EID clinics at the health facilities completed the questionnaire in Kobo collect app on tablet computers. Data was transferred from the tablet computers to the Kobo server on a daily basis. We downloaded this data from the server into Excel sheets, cleaned and imported it into Stata version 14 for analysis. Duplicate entries were removed in Stata using the exposed infant number and health facility name. HEIs missing dates for a step in the care cascade were excluded in the analysis for that particular step.
Data analysis
In univariable analysis, we presented summary statistics for all variables. Categorical variables were presented as frequencies and proportions whereas continuous variables were described using medians and interquartile ranges.
We compared time-to-sample collection, results receipt at the clinic and by the care giver and ART initiation between pre-POC and post-POC periods using the Wilcoxon rank-sum test and Kaplan- Meier curves. The log rank test was used to test for differences in time to ART initiation between the pre-POC period and the POC period as displayed in Kaplan Meier curves.
Ethical considerations
A non-research determination form was submitted to US CDC for clearance before the commencement of the evaluation as a requirement. The Office of the Associate Director for Science, U.S. Centers for Disease Control and Prevention cleared this evaluation as non-research. In the districts, we sought permission from the District Health Officers and the executive directors of the health facilities to retrieve data. We obtained verbal consent from health facility EID clinic In-charges before retrieving data. During data collection, we used infant identification numbers and initials to protect their confidentiality. We stored the data in password-protected computers.
Results
Testing characteristics of the HIV-exposed infants
This study assessed turnaround time from sample collection to receipt of results by the clinic and care giver, as well as ART linkage before and after the implementation of POC EID testing.
A total of 4,004 HIV exposed infants’ data were extracted, 1,688 (42%) from the pre-POC period and 2,316 (58%) from the POC period. Of these, 94% (3,762/4,004) had a first DNA PCR test done and 97% of these (3,667/3,762) had results. Sixty-nine infants tested positive for HIV giving an overall HIV positivity rate of 1.9 % (69/3,667) and was similar in the pre- and post- POC periods. Nine (9/65, 14%) HIV positive infants died while on ART. Sixty-five (94%) HIV positive infants in this study were initiated on ART (Figure 1).
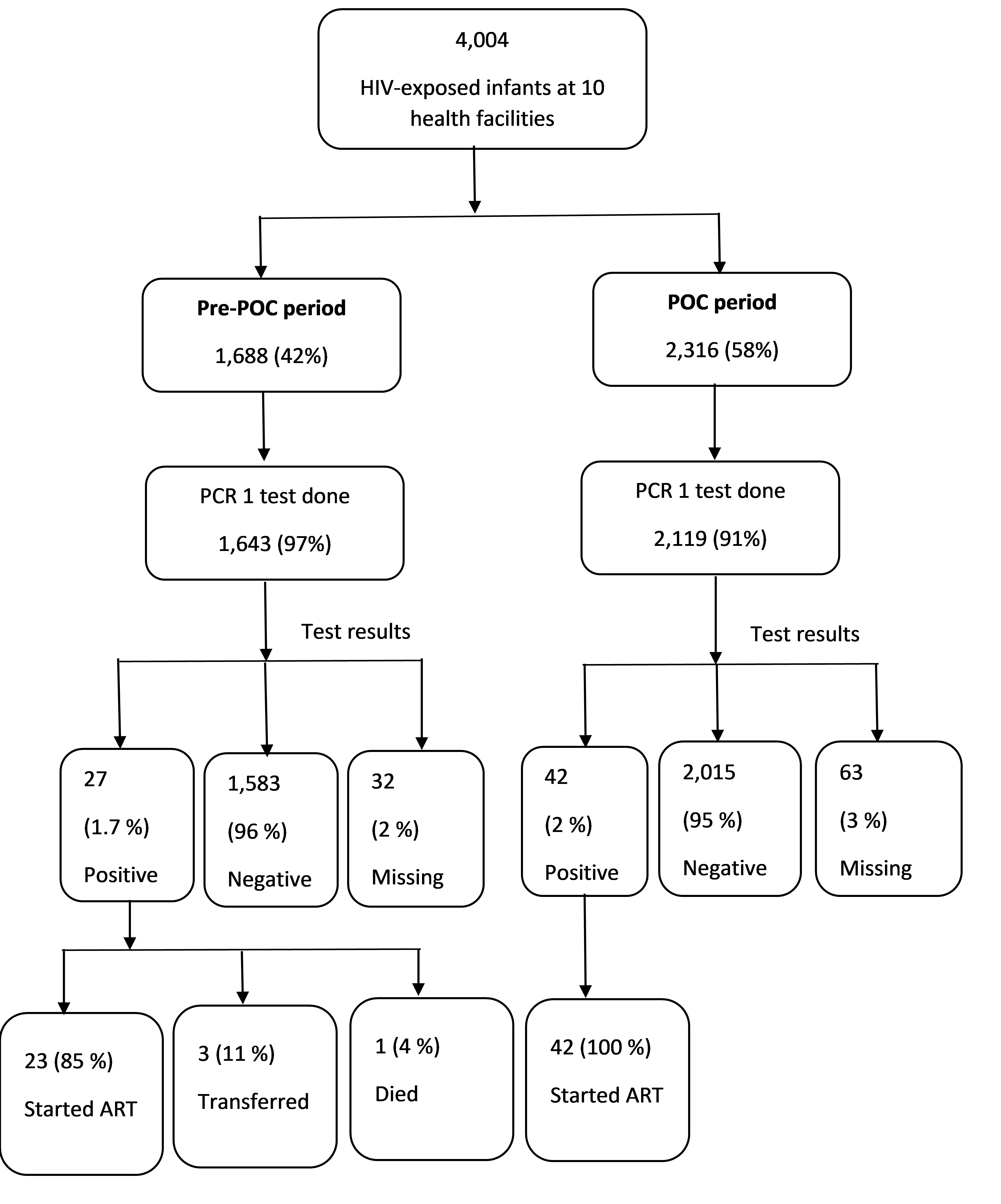
Overall, there was an almost equal proportion of males and females (47% vs 48%) with 83% (3,118/ 3,762) of HEIs being aged less than 60 days (2 months) at the time of sample collection. Thirty percent (1,081/3,615) of the HEIs received their results at the clinic on the same day of sample collection; in the POC period, 44% (924/2,057) of results were returned to the clinic on the same day of sample collection while for the pre-POC period, this proportion was 10% (157/1,610) (p<0.001). The proportion of infants’ caregivers who received results on the same day of sample collection was 40% in the POC period versus 6% in the pre-POC period (p<0.001). The proportion of HEIs caregivers who received their first DNA PCR test results within 28 days of sample collection increased from 50 % in the pre-POC period to 79% in the POC period (p<0.001) (Table 1).
The sub-group analysis showed that pre-POC, 0% (0/23) of HIV-positive infants started ART on the sample collection day compared to 40% (17/42) during POC; ART linkage by 60 days after sample collection was 91% (21/23) pre-POC and 100% (42/42) during POC (p<0.001) (Table 1).
Table 1: Socio-demographic and testing characteristics of HIV exposed infants at ten health facilities pre and post-point of care initiation, Uganda, April 2018-Sep 2021
| Total | Pre-POC | POC | ||
| Characteristic | Frequency (%) | Frequency (%) | Frequency (%) | p-value |
| Sex (n= 4,004) | ||||
| Male | 1,894 (47) | 853 (51) | 1,041 (45) | <0.001 |
| Female | 1,918 (48) | 810 (48) | 1,108 (48) | |
| Missing | 183 (5) | 25 (1) | 167 (7) | |
| Health facility level (n=4,004) | ||||
| Health centre IV | 763(19) | 346(21) | 417(18) | 0.080 |
| Hospital | 1,698(42) | 688(41) | 1,010(44) | |
| Regional referral hospital | 1,543(39) | 654(39) | 889(38) | |
| Age at sample collection(days) (n=3,762) | ||||
| Less than 60 | 3,118(83) | 1,355(82) | 1,763(83) | <0.001 |
| 60-180 | 516(13) | 257(15.6) | 259(12) | |
| 181-365 | 105(3) | 25(2) | 80(4) | |
| >365 | 23(1) | 6(0.4) | 17(1) | |
| Age | ||||
| Median (IQR) | 43(34-51) | 44(38-52) | 42(33-50) | <0.001 |
| Time from sample collection to result receipt at clinic (days) (n= 3,615) | ||||
| same day (0) | 1,081 (30) | 157 (10) | 924 (46) | <0.001 |
| 1 to 7 | 598 (17) | 192 (12) | 406 (20) | |
| 8 to 28 | 1,242 (34) | 789 (50) | 453 (22) | |
| 29 to 60 | 547 (15) | 370 (23) | 177 (9) | |
| > 60 | 147 (4) | 84 (5) | 63 (3) | |
| Time from sample collection to result receipt by care giver (days) (n= 3,503) | ||||
| same day (0) | 865 (25) | 89 (6) | 776 (40) | <0.001 |
| 1 to 7 | 618 (18) | 188 (12) | 430 (22) | |
| 8 to 28 | 835 (24) | 500 (32) | 335 (17) | |
| 29 to 60 | 749 (21) | 496 (32) | 253 (13) | |
| > 60 | 436 (12) | 284 (18) | 152 (8) | |
| HIV positive infants | ||||
| Age at sample collection(days) (n=69) | ||||
| Less than 60 | 18 (26) | 9 (32) | 9 (22) | 0.240 |
| 60-180 | 35 (51) | 16 (57) | 19 (46) | |
| 181-365 | 11 (16) | 2 (7) | 9 (22) | |
| >365 | 5 (7) | 1 (4) | 4 (8) | |
| Age | ||||
| Median (IQR) | 100(58-166) | 92(45-120) | 127(74-206) | 0.030 |
| Time from sample collection to ART initiation (days) (n=65) | ||||
| same day (0) | 17 (26) | 0 (0) | 17 (40) | <0.001 |
| 1 to7 | 15 (23) | 2 (9) | 13 (31) | |
| 8 to 28 | 22 (34) | 13 (57) | 9 (21) | |
| 29 to 60 | 9 (14) | 6 (26) | 3 (7) | |
| > 60 | 2 (3) | 2 (9) | 0 (0) | |
| Time from result receipt by caregiver to ART initiation (days) (n=65) | ||||
| same day (0) | 50 (77) | 19 (83) | 31 (74) | 0.720 |
| 1 to7 | 11 (17) | 3 (13) | 8 (19) | |
| 8 to 28 | 4 (6) | 1 (4) | 3 (7) | |
| Table 2: Turnaround times (days) from sample collection to clinic and caregiver receipt of results before and after roll out of point of care early infant diagnostic testing, Uganda, April 2018- September 2021 | |||||
| n | Median (IQR) days | p value | |||
| All HIV exposed infants | Total | Pre-POC | POC | ||
| Sample collection to results receipt at clinic | 3,615 | 12(0-26) | 20(11-31) | 1(0-18) | <0.001 |
| Sample collection to results receipt by caregiver | 3,510 | 18(1-35) | 29(16-54) | 1(0-28) | <0.001 |
| Age at result receipt by caregiver | 3,510 | 66(45-93) | 74(57-104) | 50(40-81) | <0.001 |
| HIV positive infants | |||||
| Sample collection to results receipt at clinic | 69 | 1(0-19) | 18(4-29) | 0(0-3) | <0.001 |
| Sample collection to results receipt by caregiver | 69 | 1(0-23) | 22(4-30) | 0(0-3) | <0.001 |
| Sample collection to ART initiation | 65 | 6(0-25) | 23(8-33) | 1(0-12) | 0.001 |
| Age at result receipt by caregiver | 69 | 117(74-184) | 106(73-157) | 133(75-212) | 0.340 |
| Age at ART initiation | 65 | 135(77-206) | 135(74-178) | 133(77-224) | 0.260 |
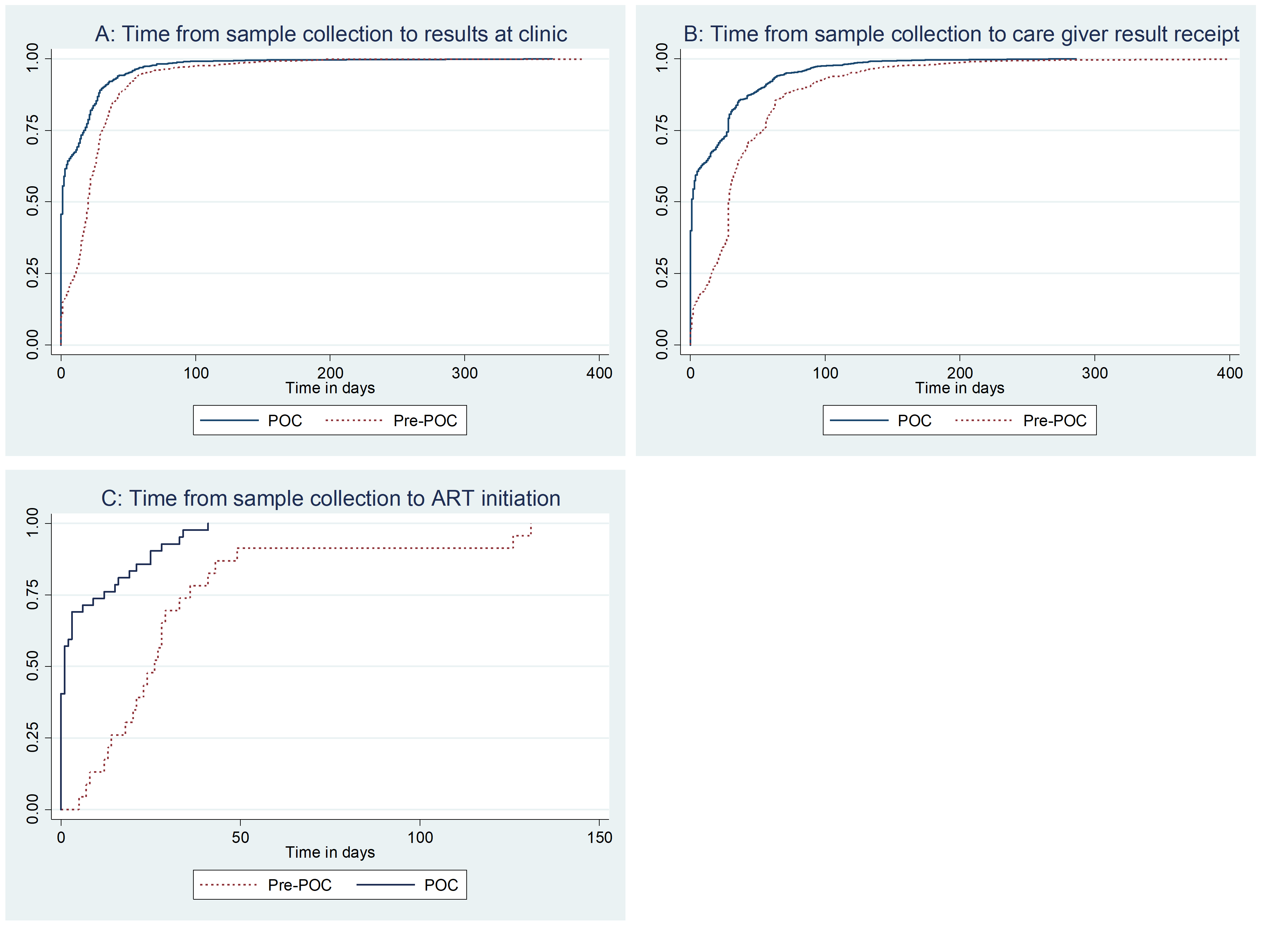
The overall median time from births to sample collection was 43 days (IQR 34-51) (p<0.001). Median time from sample collection to results receipt by the caregiver decreased from 29 days (IQR 16-54) in the pre-POC period to 1 day (IQR 0-28) during the POC period (p <0.001). The median number of days from birth to sample collection for HIV positive infants was 92 (IQR 45-120) in the pre-POC period while in the POC period, it was 127 (IQR 74-206) days (p=0.03). The median time from sample collection to ART initiation decreased from 23 days (IQR 8-33) in the pre-POC period to 1 day (IQR 0-12) in the POC period (p<0.001) (Table 2).
The time from sample collection to results receipt at the clinic in the POC period was statistically different from that in the pre-POC period (log rank p<0.001). The time from sample collection to results receipt by care giver in the POC period was statistically different from that in the pre-POC period (log rank p<0.001). The difference between time from sample collection to ART initiation for HIV positive infants before and after introduction of POC at health facilities is statistically significant (log rank p< 0.001) (Figure 2).
Discussion
In this evaluation, the novel point-of-care EID technology improved turnaround times from sample collection to results return to the clinic and caregiver, as well as linkage to ART for HIV positive infants. Of all HEIs, 83% had their first DNA PCR test done within 2 months of age. The median test turnaround time from sample collection to results at the clinic decreased from 20 days in the pre-POC period to 1 day in the POC period. The proportion of HIV infected infants was 1.9 % all of whom were linked to ART. All HIV positive infants in the POC period were initiated on art within 60 days after sample collection compared to 91% in the pre-POC period and the difference was statistically significant. This study provides information on performance of point-of-care EID testing technology at public health facilities following it’s roll out.
The findings from this study of faster turnaround times and ART initiation rates following the introduction of POC testing are consistent with those from previous studies in other African countries (8-10). We found that 94% of HEIs had a first DNA PCR test done and 83% of them received testing within 2 months of age. These proportions are greater than the 88% of HEIs that had an EID test and 74% who had their first DNA PCR within 2 months of age reported in the national annual joint AIDs review in 2021(11). This suggests EID program improvement towards attainment of the 95-95-95 2030 UNAIDS fast track target of having 95% of all people living with HIV knowing their status, 95% of all people diagnosed with HIV receiving antiretroviral therapy (ART), and 95% of all people on ART achieving viral suppression.
Notably, infants that tested positive for HIV had their first DNA PCR sample collected at a higher median age of 100 days compared to that of negative infants with a median age of 43 days at sample collection. Later age at testing among infants who turn HIV-positive suggests missed opportunities in identifying HIV-exposed infants.
The proportion of HEIs results returned at the clinic and given to care giver on the same day of sample collection was lower in this study compared to studies elsewhere. This was due to early programmatic challenges reported at the health facilities reported that included stock outs of cartridges and breakdown of the machines, which caused facilities to revert to conventional EID testing during such times.
HIV prevalence in this study of 1.9% is similar to the positivity rate of 1.7% documented in the 2021 Uganda annual AIDS review (11). HIV positivity in the POC period was slightly higher than in the pre-POC period at 2% versus 1.7% respectively. It differs from what Mwenda, Fong (9) found in Malawi where HIV positivity was 5.7% in the POC arm and 3.2% in the baseline arm.
In this study, all HIV positive infants after POC roll out were initiated on ART within 60 days after sample collection compared to 91% in the pre-POC period. This demonstrates progress in ART linkage of HEIs given that in 2021, children aged 0-9 years had the lowest ART coverage of 60% compared to other age groups (11). In contrast, in Mozambique, 90% of HIV positive infants were initiated on ART by 60 days during POC compared to 13% under conventional testing(8).
In Malawi, twice as many HIV positive infants were initiated on ART in the POC arm compared with the baseline arm (9). While POC expansion in Uganda could further improve ART linkage and loss to follow-up, there’s need to examine barriers surrounding the POC target of initiating ART on the sample collection day.
Limitations
This study used secondary data. Some data points were missing due to lack of documentation at some of the health facilities. This could have led to under estimation of the magnitude of the outcomes. In addition, some health facilities reported stock out of cartridge for POC testing and breakdowns of the machines sometimes. During such periods, they reverted to conventional EID testing. This might have led to misrepresentation of the turnaround times in this study.
Conflict of interest
The authors declare that they had no conflict of interest.
Acknowledgments
The authors would like to thank the incharges and staff at EID clinics of the following hospitals: Mityana (Mawanda Florence), Fort Portal (Festo Tugume, Merika Birungi), Mubende (Nayebare Fortunate), Kawempe (Sarah Kamya), Kiboga (Yulita Nakazibwe, Thomas Ouma), Lyantonde (Grace Nabisubi), Kyenjojo (Vincent Tuhairwe), Kyegegwa (Jonan Tumusiime, Chris Atwooki), and Ssembabule (Sophie Kawooya) for supporting the data collection.
Copyright and licensing
All materials in the Uganda National Institute of Public Health Quarterly Bulletin is in the public domain and may be used and reprinted without permission; citation as to source; however, is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
-
UNAIDS. UNAIDS Data 2020 2020 [Available from: https://www.unaids.org/sites/default/files/ media_asset/2020_aids-data-book_en.pdf.
-
UAC. Uganda AIDS Country Progress Report July 2017 to June 2018. Uganda: Uganda AIDS Commission; 2018.
-
MOH. Consolidated Guidelines for Prevention and Treatment of HIV in Uganda. Uganda: Ministry of Health; 2016.
-
WHO. WHO Recommendations on the diagnosis of HIV infection in infants and children. Geneva, Switzerland: World Health Organization; 2010.
-
WHO. HIV diagnosis and ARV use in HIV-exposed infants: a programmatic update. Geneva, Switzerland: World Health Organization; 2018.
-
WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland World Health Organization; 2016.
-
WHO. Updated recommendations on HIV prevention, infant diagnosis, Antiretroviral initiation and monitoring. 2021.
-
Jani IV, Meggi B, Loquiha O, Tobaiwa O, Mudenyanga C, Zitha A, et al. Effect of point-of-care early infant diagnosis on antiretroviral therapy initiation and retention of patients. Aids. 2018;32(11):1453-63.
-
Mwenda R, Fong Y, Magombo T, Saka E, Midiani D, Mwase C, et al. Significant patient impact observed upon implementation of point-of-care early infant diagnosis technologies in an observational study in Malawi. Clinical Infectious Diseases. 2018;67(5):701-7.
-
Boeke CE, Joseph J, Wang M, Abate ZM, Atem C, Coulibaly KD, et al. Point‐of‐care testing can achieve same‐day diagnosis for infants and rapid ART initiation: results from government programmes across six African countries. Journal of the International AIDS Society. 2021;24(3):e25677.
-
Commission UA. Annual Joint AIDS Review Report 2020/21. 2021.


Comments are closed.