Yellow Fever Vaccine should be Introduced in Uganda’s Routine Immunization Schedule Policy Brief
Authors: Maureen Nabatanzi1, Benon Kwesiga1, Gloria Bahizi1, Lilian Bulage1, Bernard Lubwama2, Alex Riolexus Ario1 Affiliations: 1Uganda Public Health Fellowship Program, Kampala, Uganda 2Ministry of Health, Kampala, Uganda
Summary
Despite the availability of an effective vaccine, Uganda continues to experience yellow fever outbreaks. On 8 May 2019, the Minis- try of Health confirmed a yellow fever outbreak in Masaka District among children (4 cases, 0 deaths). Although Uganda held reactive vaccination campaigns in central and southwestern districts – including Masaka in 2016, pockets of the population remain that are not vaccinated. Between May 2019 and February 2020, four more outbreaks were reported in four separate districts (9 cases, 6 deaths). We recommend integration of yellow fever in the routine immunization schedule for children to ensure all Ugandans are vaccinated to prevent outbreaks.
Introduction
Yellow fever is an acute viral hemorrhagic disease caused by yellow fever virus. Most infected persons are asymptomatic. Initial symptoms include sudden onset of fever, chills, head- ache, backache, general muscle pain, fatigue, nausea, and vomiting. In approximately 15% of infected persons, a brief remission for less than a day is followed by recurrence of initial symptoms and progression to jaundice and hemorrhage. Among these severe cases, 20-50% die (1, 2).
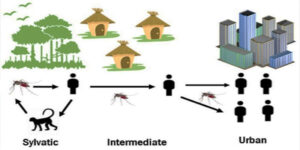
Both monkeys and humans can be infected with yellow fever virus, which has a sylvatic (jungle) cycle, an intermediate cycle, and an urban cycle. In the sylvatic cycle of transmission, mosquitoes acquire the virus by feeding on infected monkeys and transmit it to persons working or living around the forest. In the intermediate cycle, the virus is transmitted person-to- person in forest-bordering areas. The virus can then enter an urban cycle where it is transmitted from person-to-person in areas with high mosquito density and where most people are unvaccinated (2, 3). Yellow fever occurrence is influenced by the presence of the Aedes mosquito vector, the proximity of infected monkeys, the environment, and the human population. These dynamics in turn influence the transmission cycle (Figure 1). A total of 27 African and 13 Latin American countries report a few hundred cases annually. These countries, in which yellow fever is endemic, are the most vulnerable to yellow fever outbreaks (1, 3, 4).
The yellow fever vaccine is safe and effective, and one shot confers lifelong protection to recipients. In endemic countries, World Health Organization (WHO) recommends the combined use of yellow fever vaccine through the routine Expanded Program on Immunization (EPI) and mass vaccination campaigns as an effective approach to prevent yellow fever and control outbreaks (1). A vaccine coverage of over 80% is necessary to interrupt local transmission and achieve herd immunity (4).
Uganda is one of 27 African countries with both the mosquito vector and potential monkey hosts present, and therefore at risk of yellow fever transmission (3, 5). From 1941 to 2016, Uganda reported seven yellow fever outbreaks. One of these outbreaks occurred in northern Uganda in 2010 and affected 181 case- patients, of whom 45 died (Case Fatality Rate, CFR=25%). The 2016 outbreak was reported in Masaka, Rukungiri, and Kalangala districts in central and southwestern Uganda. It affected 32 case- patients, of whom 7 died (CFR= 22%) (6, 7).
On 8 May 2019, the Ministry of Health (MoH) confirmed an out- break of yellow fever in Masaka District. The index case-patient was a 12-year-old female from Bukakata sub-county. Four more case-patients (2 probable, and 2 suspect) were identified in Buka- kata; all were children 3 to 17 years old.
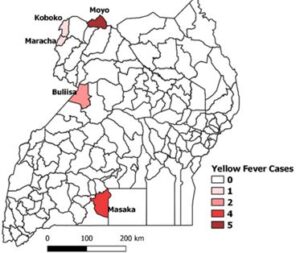
Between 8 May 2019 and 4 February 2020, four additional out- breaks of yellow fever were confirmed from Koboko (n=1; 1 con- firmed, CFR=0%), Buliisa (n=2; 2 confirmed, CFR=50%), Moyo (n=5; 3 confirmed, 2 probable, CFR=100%) and Maracha (n=1; 1 confirmed, CFR=0%) (Figure 2).
Risk factors in all affected districts were living in or working near forests inhabited by monkeys and Aedes mosquitoes, and being unvaccinated. Indeed, none of the case-patients reported in 2019 ad 2020 had received yellow fever vaccine.
Context and Importance of the problem
Uganda’s Integrated Disease Surveillance and Response (IDSR) guide- lines recommend the strengthening of routine yellow fever vaccination and reactive mass vaccination if a single case
is confirmed (5). However, Uganda has not introduced the yellow fever vaccine into the routine EPI. Following the 2016 outbreak, a reactive vaccination campaign was conducted in the affected districts with the aim of preventing further outbreaks. According to administrative records, the post vaccination coverage in affected districts was 75%. All case-patients affected by the 2019-2020 outbreaks were unvaccinated. In addition to being unvaccinated, living close to swampy and forested areas inhabited by monkeys was a risk factor. Unvaccinated Ugandans are at continued risk of yellow fever transmission. There is need to build the population’s immunity against yellow fever through introduction of the vaccine in routine EPI.
Critique of policy options
Yellow fever is a priority disease under Uganda’s IDSR. Prevention of yellow fever is mainly focused on surveillance and response activities. This is done through national weekly surveillance reports per health facility and through community-based disease surveillance (5). In addition, UVRI set up sentinel sites specific to alerts of arboviral infections. However, there is a gap in vaccination interventions.
Among the 27 African countries at high risk of yellow fever, Uganda is one of only five that has yet to introduce the vaccine into the routine EPI. According to Uganda’s immunization policy (2012), vaccination against yellow fever may be carried out by the Uganda National Expanded Program for Immunization (UNEPI) in partnership with the private sector, as guided by the disease epidemiology. The few mass vaccination campaigns conducted in Uganda occurred in selected districts and were in reaction to outbreaks. Under the International Health Regulations, Uganda necessitates all travellers to and from Uganda to possess proof of yellow fever vaccination. Ugandans and Internationals who wish to travel must sponsor their own yellow fever vaccination; on individual basis, the vaccine costs about USD $27. This status quo is contrary to UNEPI’s mission to ensure that every child and high-risk group is fully vaccinated with high quality and effective vaccines.
In 2017, the Uganda National Immunization Technical Advisory Group recommended introduction of a yellow fever vaccine in Uganda’s routine immunization schedule at 12 months of age (8). However, immunization services in Uganda are mainly funded by the government of Uganda with additional support from health development partners, and this intervention faces competing vaccine introduction priorities, limited political will, and financing challenges. UNEPI’s 2012-2016 Comprehensive Multi-Year Plan highlighted that the government of Uganda had a big funding gap for immunization. Continued advocacy with Parliament, Ministry of Finance and Economic Development, and other relevant authorities is needed to increase the health budget and thereby increase amounts available for new vaccines (9). Mobilization of partnerships for implementation can contribute to the funding gap. For example, GAVI the vaccine alliance supported the introduction of the rotavirus vaccine into routine EPI in Uganda in 2016. GAVI has also pledged to support high risk countries to implement yellow fever routine immunization (4).
Offering yellow fever vaccine through the routine EPI strategy has been proven as an effective strategy to improve coverage and reduce risk of outbreaks (1). It is feasible and safe to administer the vaccine jointly with other vaccines at 12 months of age (1). Above 80% vaccine coverage, the EPI strategy establishes high-level population immunity and continued routine vaccination of new birth cohorts is required to prevent outbreaks (4). According to WHO, by 2016, each dose cost an average of US$ 1.07 in public government-funded programs (1, 4).
Recommendations
There is need to strengthen yellow fever vaccination as a priority in prevention of future yellow fever outbreaks. Policy makers can man- date improvement of vaccine coverage of the population by: integrating the yellow fever vaccine into the existing routine EPI targeting all children aged 12 months.
References
- WHO. Background Paper on Yellow Fever Vaccine. 2013.
- APHA. Control of Communicable Diseases Manual. 20 ed. L.Heymann D, editor. Washington DC: American Public Health Association; 2015.
- CDC. CDC Yellow Book 2020. Mark D. Gershman JES, editor. USA: Centers for Disease Control and Prevention; 2019 August 02, 2019.
- WHO. A global strategy to Eliminate Yellow fever Epidemics 2017–2026. Geneva, Switzerland; 2018 31/10/2019.
- MoH. National Technical Guidelines for Integrated Disease Surveillance and Guidelines. In: Health Mo, editor. Kampala, Uganda2012. p. 413.
- Wamala JF, Malimbo M, Okot CL, Atai-Omoruto AD, Tenywa E, Miller JR, et al. Epidemiological and laboratory characterization of a yellow fever outbreak in northern Uganda, October 2010-January 2011. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2012;16(7):e536-42.
- Kwagonza L, Masiira B, Kyobe-Bosa H, Kadobera D, Atuheire EB, Lubwama B, et al. Outbreak of yellow fever in central and south- western Uganda, February–may 2016. BMC Infectious Diseases. 2018;18 (1):548.
- UNITAG. Prioritisation of vaccine introduction in the UNEPI. Kampala, Uganda, 2017.
- UNEPI-MoH. Comprehensive Multi-Year Plan (cMYP) 2012-2016. Kampala, Uganda, 2012.


Comments are closed.