
Dear Reader,
We take great pleasure in welcoming you to Issue 4, Volume 5 of the Uganda National Institute of Public Health (UNIPH) Quarterly Epidemiological Bulletin.
We aim to inform the district, national, and global stakeholders on disease outbreak investigations, public health surveillance, and interventions under- taken in detecting, preventing, and responding to public health events in Uganda.
In this issue, we present a variety of articles: contact tracing & community based surveillance for COVID-19 using health assistants, Masindi District; association between perceived risk infection with COVID-19 and protective behavior among adults; uptake of HPV vaccination in Uganda: barriers & opportunities; estimating the cost of managing COVID-19 patients in Uganda; cost-effectiveness and decision making analysis of national airport screening for COVID-19. We also present a policy brief on improving malaria reporting by VHTs under ICCM and training of District Health Teams on malaria normal channels.
Should you have any questions or require additional information related to articles in this bulletin please contact us on: abyaruhanga@musph.ac.ug, gamanya@musph.ac.ug, pthiwe@musph.ac.ug OR lbulage@musph.ac.ug
We hope you find this information valuable and we shall appreciate any feedback from you.
Thank You
EDITORIAL TEAM
Dr. Alex Riolexus Ario |
Director, Uganda National Institute of Public Health, MoH
Dr. Allan Niyonzima Muruta|
Commissioner, Integrated Epidemiology, Surveillance and Public Health Emergencies, MoH
Paul Mbaka|
Asst. Commissioner Health Services, Division of Health Information, MoH
Lilian Bulage |
Scientific Writer, Uganda Public Health Fellowship Program, MoH
Dr. Benon Kwesiga |
Field Supervisor, Uganda Public Health Fellowship Program, MoH
Daniel Kadobera |
Field Supervisor, Uganda Public Health Fellowship Program, MoH
Aggrey Byaruhanga|
PHFP Fellow, AIDS Control Program ,MoH
Geofrey Amanya|
PHFP Fellow, Butabika National Referral Hospital, MoH
Patricia Okumu Thiwe |
PHFP Fellow, National Malaria Control Division, MoH
Inside this Issue,
- Upcoming Events
- Automating Early Systems and detection of Outbreaks.
- Perceived Risk of Infections with Covid-19 & Protective behavior Among Adults
- Contact tracing and Community-based Surveillance for Covid-19 Using Health Assistants
UPCOMING EVENTS
1. Eliminating NTDs: Together towards 2030 – Formal launch of the new roadmap for Neglected Tropical Diseases
On 28 January, 2021, WHO will launch its roadmap for Neglected Tropical Diseases (NTDs), ‘Ending the neglect to attain the Sustainable Development Goals: a roadmap for Neglected Tropical Diseases 2021–2030’. This is a high-level strategic document and advocacy tool, aimed at strengthening programmatic response to NTDs through shared goals and disease specific targets backed by smarter investments.
2. Updating the WHO global technical strategy for malaria 2016-2030 on 28 January, 2021
The WHO Global technical strategy for malaria 2016-2030 – adopted by Member States in May 2015 is designed to guide and support all malaria-affected countries as they work to reduce the human suffering caused by the world’s deadliest mosquito-borne dis- ease. The strategy sets four global targets for 2030, as well as interim milestones to track progress. The 2030 targets include: reducing malaria case incidence by at least 90%, reducing malaria mortality rates by at least 90%, eliminating malaria in at least 35 countries and preventing a resurgence of malaria in all countries that are malaria-free.
3. Beat Leprosy, End Stigma and advocate for Mental Wellbeing
World Leprosy Day takes place on 31 January 2021. This year, we unite around one goal, which is to Beat Leprosy. This World Leprosy Day, we invite the international community to help spread the word that Leprosy Is Curable, join in the fight to End Stigma, and advocate for the Mental Wellbeing of persons who have experienced leprosy and other neglected tropical diseases.
4. 5th Graduation Ceremony of Uganda Public Health Fellowship Program on 29th January, 2021
The Uganda Public Health Fellowship Program (UPHFP) is a tripartite public health workforce capacity building program of the Ministry of Health, Makerere University School of Public Health and the US Centers for Disease Control and Prevention. On 29th Jan, UPHFP will hold its 5th graduation ceremony. On that day 12 fellows will graduate
Contact Tracing and Community-Based Surveillance for COVID-19 Using Health Assistants, Masindi District, Uganda
Authors: Bob O. Amodan*1, Immaculate Akusekera1, Geoffrey Amanya1, Josephine Nama- yanja1, Daniel Kadobera1, Alfred Driwale2, Alex R. Ario1, 2
1 Uganda Public Health Fellowship Program, Kampala, Uganda
2 Ministry of Health Uganda, Kampala, Uganda
Summary
On 1 May 2020, the first case of COVID-19 in Masindi District, Uganda, was identified. The case-patient, a policeman, who had more than 750 contacts. Previously, central-level healthcare workers from the Ministry of Health had been deployed to conduct contact tracing in districts, which was highly resource-intensive. We set out to build capacity of health Assistants in Masindi to strengthen district COVID-19 surveillance response capacity, and compared costs of deploying central-level vs local-level responders using this case as a model.
We spent May 2-16, 2020 in Masindi District, working with the District Task Force to identify 31 environmental health workers (Health Assistants [HAs]) and train them for 2 days in COVID-19 contact tracing and community-based surveillance (CBS). We tracked the proportion of all contacts followed up by HAs each day and supported HAs to establish a CBS system comprising com- munity leaders and village health teams. We calculated and compared response costs between use of 31 HAs and 10 central-level epidemiologists for this work.
HAs identified 729 contacts, and visited or tele- phoned 20-25 contacts daily for 14 days after their last exposure to the case-patient. Of the 729 contacts, 725 (99.5%) were followed for 14 days and four were lost to follow-up. All contacts tested negative for SARS-CoV-2 at Day 14. From 5-16 May, the new CBS system received and investigated 531 separate community alerts for suspected cases unlinked to the index case.
Using HAs vs central-level epidemiologists reduced the 14-day response costs by 70% ($8,300 to $2,500). District-level training in COVID-19 con- tact tracing and CBS from the central level enabled a less costly and a more effective approach to alert response and contact tracing at the local level. Decentralized use of the HAs to conduct contact tracing and CBS can increase community and District ownership of COVID-19 response.
Introduction
On 1 May 2020, the first community transmission case of COVID-19 was identified in Masindi District, western Uganda during a rapid assessment survey among high-risk persons on the prevalence of the COVID-19 in communities. The case-patient was a 29-year-old, police officer who worked at homicide department of Masindi District as a criminal investigator. This case-patient was the first known case that had no travel history and link to truck drivers, who had contributed to bulk of confirmed cases in Uganda at the time
On 2 May 2020, the Public Health Emergency Operations Centre, Ministry of Health notified the Incident Management Team, and a team of four field epidemiology fellows from the Uganda Public Health Fellowship Program were sent to Masindi District to establish an epidemiologic linkage, and support the district in response.
At that time, Masindi District lacked the capacity to do an effective COVID-19 response. This included contact tracing, which involves contact identification and listing, and then follow-up to detect any contacts who become ill. This critical component of the COVID-19 response is facilitated by local structures and knowledge that the central level team might have a difficult time providing. In addition, Masindi did not have a community-based surveil- lance structure. This was critical for ensuring the presence of an alerts system, which can detect any suspected cases through reporting.
Contact tracing is the identification, listing, and follow-up of all exposed persons to determine whether they could have contracted the disease from their contact with the infected person. It is also one of the single most important activity that breaks the chain of transmission of COVID-19 [1]. As opposed to active surveillance, use of community-based surveillance systems to control spread of COVID-19, is an attractive alternative design and operation is recommended. Effective community-based contact tracing surveillance program requires constant community engagement [2] and use of Health Assistants. We supported the district contain the spread of the virus by building capacity of Health Assistants to strengthen surveillance, and compared costs of deploying central-level vs local- level responders (Health Assistants) using this case as a model.
Methods
Identification and training of Health Assistants
We worked with the COVID-19 District task force on 2 May, 2020 to identify 31 Health Assistants who were trained from 3-4 May 2020 on how to conduct contact tracing using WHO guidelines [2]. All contacts were listed from 20 April to 2 May, 2020 when the case was isolated for management. The start contact date of 20 April 2020 was chosen because the case-patient had reported history of cough and flu-like symptoms from 22 to 25 April, 2020.
We further trained the Health Assistants on community-based surveillance. In so doing, key issues such as: benefits of a functional community-based surveillance system, community case definition for COVID-19, and how-to set-up and manage community COVID-19 alerts was shared with Health Assistants.
Contact identification and Listing during COVID-19 outbreak, Masindi District, Uganda, May 2020
On 2nd May, 2020, we conducted a telephone interview with the case-patient to understand the list of people who had contact with him during
the specific timeframe mentioned above. Furthermore, we interviewed the officer in-charge of
the local police station to further understand the duties the case-patient was doing, and authorize release of the police documents for our perusal. We reviewed police records to identify the people who had interacted with the case-patient during execution of his duties.
With support from the district health office, the Health Assistants called upon all contacts of the case-patient through radio talk shows and announcements to volunteer themselves to the health authorities regardless of any punitive, security, and immigration issues they were facing In addition, Health Assistants made phone calls to the identified contacts and further conducted home visits to collect contact details and linked them to the quarantine management team.
Contacts were given phone calls (if having phones) and visited by the Health Assistants in the afternoons after the training to ascertain the level of contact with the confirmed case-patient. During listing, demographic, residence, exposure history, clinical and relationship with the case information was collected. Health Assistants also counselled contacts, and emphasized on pre- cautions and rationale for contact tracing. A total of 729 contacts were listed; of which, 323 were listed on 2 May, 2020 and later geographically quarantined. On 3 May, 2020, another 125 contacts were listed and later taken to institutional quarantine centers for monitoring. The other, 281 contacts were listed on 4 May, 2020 and asked to stay at home on self-quarantine.
Confidentiality of the contacts’ information was maintained and well managed by the Health Assistants. Efforts were made to have the contacts’ lives protected, and stigma arising from community members was also confronted using risk communication messages aired by Health Assistants or Health educators on radios.
Follow-up of contacts during COVID-19 outbreak in Masindi District, Uganda, May 2020
The Health Assistants opted to follow-up all the contacts for 14 days from the date the case was taken for isolation (2 May, 2020). This was so, first, because most of the contacts could not remember their exact dates of last contact with the index case-patient. Secondly, be- cause majority of the contacts had closely mixed with each other at quarantine centers and police stations.
Each Health Assistant was assigned 20 to 25 contacts to follow-up on daily basis using the Ministry of Health’s COVID-19 follow-up form. The team either home visited or telephoned the contacts to understand whether they had developed signs and symptoms in each of the 14 days of observation. Only 11 Health Assistants were given infrared thermo scans for taking temperature readings, and other 20 took self-reported fever since the thermometers were not enough.
Health Assistants reported to the District Surveillance Focal person on daily basis, specifically highlighting number of contacts followed-up, lost to follow-up, and those who developed signs and symptoms related to COVID-19. Contacts who developed COVID-19 related signs and symptoms were isolated and immediately tested for COVID-19.
We entered data in excel spreadsheets, analyzed on daily basis into descriptive statistics, and shared with the COVID-19 District rapid response team and District task force for decision making.
Activating community-based surveillance during COVID-19 outbreak, Masindi District, Uganda, May 2020
Health Assistants set-up a functional community-based surveillance system from 5 to 16 May 2020. They reached out to the village health teams and local council one leaders to educate them on the urgent need to control COVID-19 by opening channels for reporting of any suspected cases to the district health authorities. Health Assistants set-up a functional community-based surveillance system from 5 to 16 May 2020. They reached out to the village health teams and local council one leaders to educate them on the urgent need to control COVID-19 by opening channels for reporting of any suspected cases to the district health authorities.
Additionally, Health Assistants also shared COVID-19 community case definition with the local leaders so that they knew what to look for, and left their phone numbers with them for reporting of any persons who met the community case definition.
Costing the response during COVID-19 outbreak, Masindi District, Uganda, May 2020
We also roughly calculated the costs of all the activities we carried out using the Health Assistants, and compared them to what the costs would have been if we had only used central- level health workers. These costs included Safari day allowances for HAs or per diem for central level health workers and fuel.
Results
Contacts traced during COVID-19 out- break, Masindi District, Uganda, May 2020
A total of 729 contacts were listed and followed-up for 14 days. The first 3 days in figure 1 showed zero performance in terms of follow- up. The proportions of contacts followed-up by either home visit or phone call increased from day 4 at 94% to day 14 at almost 100%. The proportion of contacts followed-up by only home visit also gradually increased from 43% to 94% (Figure 1).
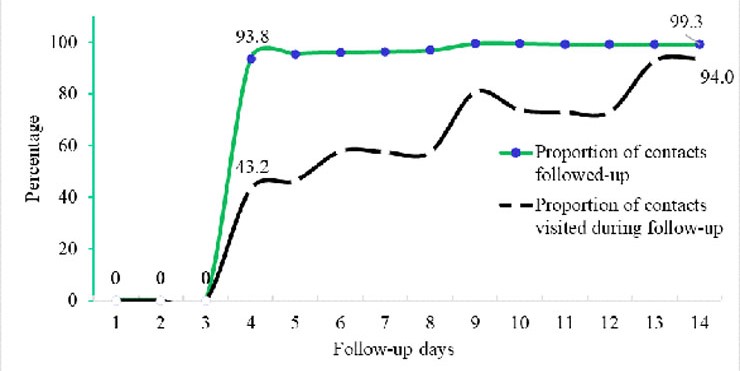
In addition, during 14-days follow-up, no contact turned positive for COVID-19 even when 3.8% (28/729) contacts developed signs and symptoms related to COVID-19. Only 0.55% (4/729) contacts were lost to follow-up in between day 9 and 13.
Activating community-based surveillance during COVID-19 outbreak in Masindi District, Uganda in May 2020
Health Assistants also managed to set up a functional community-based surveillance system. As compared to before, when there were no formal COVID-19 alert management and linkage systems, 531 non-index case-linked community alerts were collected and investigated in between 5-16 May, 2020.
Response costs that were incurred during COVID-19 outbreak in Masindi District, Uganda in May 2020
The cost doing contact tracing and community-based surveillance when central-level team is used would be over $8,300 and that of Health Assistants would be 2,500 US dollars (Table 2).
The difference between separate costs of central-level team and Health Assistants response would be over USD 5,800, which is a 70% reduction of costs if we used only health assistants.
Table 1: COVID-19 Response costs of using central-level versus Health Assistants during COVID-19 outbreak, Masindi, Uganda, May 2020
| Item | Qty | Unit cost | Freq | Total Cost (UgX) | Total Cost (USD)** | |||
| Allowance (per diem)- MoH | 5 | 160,000 | 15 | 12,000,000 | 3,158 | |||
| Fuel | 2 | 60,000 | 15 | 1,800,000 | 474 | |||
| Driver allowance | 2 | 160,000 | 15 | 4,800,000 | 1,263 | |||
| Allowance (per diem)- UPHFP | 5 | 150,000 | 15 | 11,250,000 | 2,961 | |||
| Driver allowance | 1 | 75,000 | 15 | 1,125,000 | 296 | |||
| Fuel | 1 | 60,000 | 15 | 900,000 | 237 | |||
| Sub-total -1 | 31,875,000 | 8,388 | ||||||
| Use of Health assistants (costing) | ||||||||
| Allowance (SDA) | 31 | 12,000 | 14 | 5,208,000 | 1,371 | |||
| Fuel | 31 | 10,000 | 14 | 4,340,000 | 1,142 | |||
| Subtotal-2 | 9,548,000 | 2,513 | ||||||
** USD 1.0 = 3,800 UgX
DISCUSSION
This activity built the capacity of Health Assistants in contact tracing and community-based surveillance. In addition, costs of deploying central-level vs local-level responders (HAs) using this case as a model were estimated. The Health Assistants have a broad spectrum and all encompassing range of skills that makes them unique and easily understand a lot of disciplines within prevention and control of outbreaks [7]. The capacity of Health Assistants was greatly built with less costs, and turned to be a more effective approach for implementing contact tracing and community-based surveillance.
This case-patient generated the highest number of documented contacts in Uganda. This could be partly due to the nature, high mobility, and busy schedule of the case-patient. High mobility patterns or habits have been linked to high COVID-19 transmission [8, 9].
The contacts were not followed-up as expected in the first three days because the Health Assistants were being mobilized and trained on the essentials of contact tracing and community-based surveillance. In the subsequent days (from day 4 to 14 of follow-up), the Health Assistants were deployed under our supervision. Overall follow-up by either home visit or phone call was well done as exhibited by the high daily proportion of contacts followed-up from the first to the last day. This could be because Health Assistants were familiar, well known, and respected health workers, and thus did not have to grapple with local mistrust, language barrier, terrain, and culture of Masindi District. Whereas daily contact follow-up was not 100% as expected [6, 10], this achievement was way too high when we consider Uganda’s possible past challenges in contact follow-up such as, non-cooperation of contacts, poor geographical and settlement patterns.
To note, there was progress in terms of proportion of contacts home visited during follow-up even when the team did not make it to the recommended 100% coverage on each day. This was attributed to wide dispersion between homes of self-quarantined contacts in the rural areas, and that there was inability to trace contacts in the urban areas in the maiden days of follow-up. In addition, inaccurate and miss leading residence data was collected by the local untrained health workers who had partly done contact listing.
Even when the institutional quarantine centre had registered a run way, a loss to follow-up of any contact was not constituted because, by the help of the police, community members and media houses, the escapee was apprehended and returned to the centre within 1 day. This escape happened because; first, the parameter wall was too low, so one could easily jump over, and secondly, the security personnel were not many enough. Later, the security at the quarantine centre was beefed up by the army as recommended by the country’s quarantine guidelines [11], and that explained why we did not have any other escapees during the follow-up days. At self-quarantine homes, there was a loss to follow-up that could be attributed to indiscipline (not to staying home) among contacts or feeling of stigma because of health-worker home visit follow-up. In addition, some of the contacts were bodaboda motorcycle riders who wanted to fend for themselves and families. Even when efforts were made to involve the local leadership to make sure that all contacts adhered to the self-quarantine regulations, those lost to follow-up went to stay somewhere else unknown until the 14 days follow-up was over. Escapes and loss to follow-up could cause spread of COVID-19 among other groups of people. Use of digitalized contact tracing could reduce possible stigma due to face-to-face contact interactions with the contact tracing team [12].
No single contact, even those who developed signs and symptoms related to COVD-19 turned positive. This could partly be because the case was not exposed to the contacts during his most infectious stage of the infection since he developed COVID-19 related signs and symptoms after he was isolated. The risk of transmission of COVID-19 is higher when the case is symptomatic [13, 14].
Community-based surveillance systems were established and kept functional during the response to the outbreak. This is partly due to the fact that Health Assistants have a sound technical training, respect from the local leaders, since they often work together in many other health programs at community level.
Conclusion and Recommendations
In conclusion, building capacity of Health Assistants enabled proper contact listing and follow-up. Use of Health Assistants to conduct contact tracing and Community based surveillance activities can increase community and district ownership of COVID-19 response. In addition, Use of HAs was a less costly and a more effective approach to alert management and contact- tracing.
Since the outbreak was likely to take long or even have other waves, we recommended that the Ministry of Health, Uganda adopts using Health Assistants to accomplish the demands of COVID-19 contact tracing and community-based surveillance. In adopting the use of health assistants, there need to: Build their capacity, support digital innovations of contact tracing, fund the tasks and monitor performance.
References
- Naming the coronavirus disease (COVID-19) and the virus that causes it [https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it]
- MoH: Uganda confirms 1st case of COVID-19. Saturday 21 March 2020. In.: Ministry of Health, Uganda; 2020.
- Liu J, Liao, X., Qian, S., Yuan, J., Wang, F., Liu, Y., … Zhang, Z.: Community transmission of severe acute respiratory syndrome coronavirus 2. Emergency Infectious Diseases 2020, 26(6).
- Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K: Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020, 323(16):1610-1612.
- World Health Organization: Critical preparedness, readiness and response actions for COVID-19: interim guidance, 22 March 2020. In. Geneva: World Health Organization; 2020.
- World Health Organization: Contact tracing in the context of COVID-19: interim guidance, 10 May 2020. In.; 2020.
- Morse T, Chidziwisano K, Musoke D, Beattie TK, Mudaly S: Environmental health practitioners: a key cadre in the control of COVID-19 in sub-Saharan Africa. BMJ Global Health 2020, 5(7):e003314.
- Badr H, Du H, Marshall M, Dong E, Squire M, Gardner L: Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study. The Lancet Infectious Diseases 2020.
- Cartenì A, Di Francesco L, Martino M: How mobility habits influenced the spread of the COVID-19 pandemic: Results from the Italian case study. Science of The Total Environment 2020, 741:140489.
- Africa Centres for Disease Control and Prevention: Guidance on Contact Tracing for COVID-19 Pandemic. In.; 2020.
- Ministry of Health: Guidelines on Quarantine of Individuals in the Context of Containment of Coronavirus Disease (COVID-19) in Uganda. In. Kampala; 2020.
- Owusu PN: Digital technology applications for contact tracing: the new promise for COVID-19 and beyond? Global Health Research and Policy 2020, 5(1):36.
- Cheng H-Y, Jian S-W, Liu D-P, Ng T-C, Huang W-T, Lin H-H, Team ftTC-OI: Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Internal Medicine 2020, 180(9):1156-1163.
- Lipsitch M, Cohen T, Cooper B, Robins JM, Ma S, James L, Gopalakrishna G, Chew SK, Tan CC, Samore MH et al: Transmission Dynamics and Control of Severe Acute Respiratory Syndrome. Science 2003, 300(5627):1966.
Association Between Perceived Risk of Infection With COVID-19 And Protective Behavior Among Adults in Uganda, May 2020
Authors: Josephine Namayanja1, Immaculate Akusekera1, Patricia Thiwe1, Elizabeth Katana1, Steven N Kabwama1,
Affiliations
1Uganda Public Health Fellowship Program, Ministry of Health, Kampala, Uganda
*Corresponding author: jnamayanja@musph.ac.ug
Summery
Since the confirmation of the first SARS-CoV-2 infection in Uganda on March 21, 2020, government efforts to minimize spread emphasized individual protective behaviors such as frequent hand washing, using alcohol-based hand sanitizers, social distancing, and wearing face masks in public. We assessed individual risk perception of COVID-19 infection and protective behavioral responses early in the outbreak to inform interventions to reduce COVID-19 spread. We conducted an online survey during April 27-May 2, 2020 and distributed it via social media. Respondents were obtained using a quasi- snowball strategy. We asked about perceived risk of contracting COVID-19 and individual preventive behavioral activities implemented since March 21. We performed modified Poisson regression analysis to identify factors associated with perceived risk of contracting COVID-19.
Amongst 430 respondents (mean age=37 years, SD±11.8), 217 (51%) were males, 344 (80%) were university-educated and 199 (46%) had >5 household members. Nearly all (97%) self-reported washing their hands regularly, and 412 (96%) believed that regular handwashing prevented COVID-19 spread; 244 (57%) reported that regular handwashing was easy for them. Although 352 (82%) believed face masks prevented spread of COVID-19, only 106 (25%) reported wearing them in public; 371 (86%) said they could not easily access masks. Additionally, 400 (93%) believed that using alcohol-based hand sanitizers would prevent COVID-19 spread but only 324 (75%) said they used them. Three hundred and forty-eight (81%) reported being worried about contracting COVID-19. Being worried about contracting COVID-19 was higher among participants who washed their hands with soap and water regularly (PR: 4.20, 95%CI: 1.3–13).
Among our survey respondents, perception of individual COVID-19 risk was high and associated with regular self-reported regular hand washing with water and soap. Few people easily accessed face masks and sanitizers and easily practiced social distancing. We recommended public health authorities to improve coverage of hand washing facilities and increase mask access.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus2 (SARS-CoV-2) (WHO, 2020). The virus is mainly spread during close contact, and by small droplets produced when people cough, sneeze, or talk. People may also catch COVID-19 by touching contaminated surfaces (WHO, 2020).
Since no specific vaccines or treatments have been developed, avoiding exposure is considered to COVID19 is the best available way to prevent the infection with this virus. The most important protective measures on a personal level included frequent hand washing, using alcohol-based hand sanitizers, maintaining distance of at least 2m with other people when in a public place, wearing a face mask when in public, avoiding touching nose, mouth and eyes (WHO,2020). To achieve the successful implementation of such measures recommended by public health authorities, the willingness of the public plays an important role (Ibuka Y; et al, 2009).
Since the confirmation of the first case of COVID-19 on March 21st, 2020, the government of Uganda instituted various preventive measures to reduce the chances of spread of the disease. However, the public’s perceptions of risk of the disease and the extent of implementation of the recommended preventive measures was not clearly understood. We assessed the public’s risk perception for COVID-19 and protective behavioral response towards the disease so as to generate evidence for targeted development of interventions against COVID19.
METHODS
Design and setting
We conducted a self-administered online survey designed on Google forms between 27 April and 2 May 2020. By the time the survey was conducted, 79 cases of COVID-19 had been reported in Uganda, with 46 recoveries and no death. By 2 May 2020, when the survey was concluded, Uganda had 88 COVID-19 confirmed cases with 52 recoveries and no death had been recorded.
Study participants and data collection
All adult people from the general public were invited to participate in this survey. The URL link for the survey material was https://docs.google.com/forms/d/e/1FAlpQLScS-0PIT-ESmj5YOxuVC-sFo-bVPrKZm-bQDd23xqcTuTepvw/viewform?vc=0&c=0&w=1). UWe used social networking sites such as, WhatsApp and Facebook to share the survey link with potential participants. We collected data on;
Survey tool
The first part of the survey questionnaire included information about socio-demographic characteristics of the participants, such as age, gender, occupation, highest level of education, household size and District of residence.
The second part included questions to ascertain peoples’ perception of risk for COVID-19. The questions were mainly about peoples’ knowledge about COVID-19, how worried they were about the disease, whether it was possible for them to contract the disease, whether it was possible for their loved ones to contract COVID-19, whether there was a likelihood for an infected person to pass on the infection to other people and whether they knew of any proven treatment against COVID-19.
The third part included questions that were asked to understand peoples’ preventive behavioral response against COVID-19. The questions were about regular hand washing, how easy they found practicing regular hand washing, using alcohol-based hand sanitizers, how easy it was for them to access alcohol-based hand sanitizers, wearing face mask when in public, how easy it was to access face masks, practicing social distance, how easy it was to practice social distance and whether practicing all these preventive behaviors would help prevent the spread of COVID-19.
Data analysis
The dependent variable was perceived risk for COVID-19 which was determined and categorized on two levels; not worried and worried. The independent variables considered included socio-demographics including age, sex, education, occupation and house hold size. Other factors included if they thought it was possible to contract COVID-19, if they thought they could transmit it to others, if they washed their hands with soap and water regularly, wearing face mask in public, if they practiced social distancing and overall preventive behavior for COVID-19 determined on a binary scale. We performed modified Poisson regression analysis to identify factors associated with perceived risk (worry about) of contracting COVID-19.
RESULTS
Socio demographic characteristics of the participants
A total of 430 individuals participated in this survey with a mean age of 37 years, SD of 11.8 and ranging from 20 to 67 years. The majority (217, 51%) were males, 344 (80%) had attained up to university level of education, 279(65%) were civil servants, 143 (33%) were residents of Kampala district and 199 (46%) had more than five people staying in their household.
Protective behavior for COVID-19 among the participants
Of the 430 participants, 415 (97%) washed their hands regularly, only 106 (25%) were wearing face masks in public. Use of alcohol-based sanitizers was reported by 324 (75%) and 328 (76%) would keep a 2m distance from other people when in public.
Ease of engaging in protective behavior
Two hundred forty-four (57%) of the respondents practiced regular hand washing with ease, only 59 (14%) would easily access face mask, 75 (17%) found it easy to access sanitizers, and only 44 (10%) would easily keep a 2m distance from other people.
Perceptions about the effectiveness of preventive behavior
Nearly all participants (412, 96%) believed that regular hand washing prevented the spread of COVID-19 and 352 (82%) believed that wearing a face mask could prevent the spread of the disease. Four hundred (93%) believed that using alcohol-based sanitizers would prevent the spread of COVID-19 and 388 (90%) believed that if they kept a 2m distance from other people it would prevent the spread of the disease.
Risk perception of COVID-19 among Ugandans, May 2020
Three hundred forty-eight (81%) reported being worried of contracting COVID-19.
Factors associated with perceived risk for COVID-19 among Ugandans May 2020
At bivariate analysis, the odds of being worried vs not worried about contracting COVID-19 were 4.2 times higher among participants who washed their hands with soap and water regularly (PR: 4.20, 95%CI: 1.3–13).
Discussion
The perception of risk for infection with COVID-19 was high (81%) amongst the studied individuals. Most respondents are alert to the disease progression, and adopt self-protective measures. Higher risk perception translates into the take up of more preventive measures–the more people fear, the more they protect themselves (Celine Aerts, et al; 2020).
The level of risk perception (81%) found by this study is slightly lower than the 92.5% that was reported by a study conducted in Wuhan China among 1,352 college students with aim to determine if they believed that any healthy person could contract the disease ( Ding Y, et al, 2020). The disagreement between these two study findings could be explained by the difference in the settings between China and Uganda as well as the dynamics of transmissions and progression of the disease in the two countries.
Preparedness for COVID-19 outbreak in the communities with emphasis on risk communication activities was initiated by MOH several weeks before Uganda confirmed her first case. This could have played a role in the high level of risk perception found by this study as majority of the general public were willing to take up the key preventive measures with support from the public health authorities.
These findings were similar to the findings by Dry Hurst, Sarah et al who assessed public risk perception for COVID-19 among 6,991 respondents in 10 countries across Europe and revealed a significant correlation between risk perception and the adopted preventive health behaviors ie wearing face masks, maintaining physical distance among others. In another similar study carried out in Hong Kong during the early phase of the COVID-19 Pandemic, most respondents were worried about COVID-19 (97%) (Barrios & Hochberg, 2020).
We acknowledge the limitation that the data collection approach through an Online questionnaire only targeted a certain group of people, more of the highly educated or urban dwellers, this might have over or underestimated the true risk perception of COVID-19 among Ugandans hence affect external validity of our findings. However, the findings from this study revealed that people were aware of the disease, its associated preventive measures, and exposure behaviors. It further revealed the risk perception at an individual level and this could help inform subsequent interventions and risk communication strategies as the pandemic progresses.
Conclusion
Perception of individual COVID-19 risk was high and associated with regular self-reported regular hand washing with water and soap. Few people easily accessed face masks and sanitizers and easily practiced social distancing. We recommended public health authorities improve coverage of hand washing facilities and increase mask access. Increased access to alcohol-based hand sanitizers especially in public places as well as increasing awareness regarding the implementation of individual protective behaviors is also critical. The population was unusually educated and does not represent the Uganda population at large; the study should be repeated in other settings/populations to enhance representativeness.
References
- Ibuka Y, Chapman GB, Meyers LA, Li M, Galvani AP. The dynamics of risk perceptions and precautionary behaviour in response to 2009 (H1N1) pandemic influenza. BMC Infect Dis. 2010; 10:296. https://doi.org/10.1186/1471-2334-10- 296 PMID: 20946662 PMCID: PMC2964717
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. Basic protective measures against the new coronavirus. Accessed 25 Mar 2020.
- Barrios, J. M., & Hochberg, Y. (2020). Risk perception through the lens of politics in the time of the covid-19 pandemic (0898-2937).
- Kwok, K. O., Li, K. K., Chan, H. H., Yi, Y. Y., Tang, A., Wei, W. I., & Wong, Y. S. (2020). Community responses during the early phase of the COVID-19 epidemic in Hong Kong: risk perception, information exposure and preventive measures. MedRxiv.
- Céline Aerts, Mélanie Revilla, Laetitia Duval, Krijn Paaijmans, Javin Chandrabose, Horace Cox, Elisa Sicuri (April, 2020) Understanding the role of disease knowledge and risk perception in shaping preventive behavior for selected vector-borne diseases in Guyana
Automating Early Warning System for Timely Detection of Outbreaks in Uganda; A Solution to Rapid Response and Containment of Public Health Emergencies:
-
A Policy Brief
Authors: Sandra Nabatanzi1, Benon Kwesiga1, and Alex Riolexus Ario1
1Uganda Public Health Fellowship Program, Kampala, Uganda
Summery
Delayed detection of outbreaks results into increased transmission of diseases within communities and health care facilities. Although there is improved reporting by health facilities at district level, there is no automated mechanism to quickly analyse trends of diseases against the expected number on cases in a specific time period / threshold, immediately detect surges and provide reports for verification and action. A policy on creation and use of a monitoring system or automated mechanism to quickly analyse all the data collected from the surveillance system and promptly create reports that are used for informed decision making and public health actions should be in place.
Introduction
Public health surveillance is critical for planning, implementation, and evaluation of public health practices. In Uganda, various types of surveillance are used within the national programmes including:
1) focussed location e.g. health facility and community based surveillance;
2) sentinel surveillance which is a designated health facility or any reporting site used for early warning of epidemics;
3) laboratory surveillance used for detecting events and
4) disease specific surveillance with activities aimed at targeted data for specific diseases.
In 1998, surveillance systems were weak and parallel in Africa and inefficient for outbreak preparedness and response to public health emergencies. The World Health Organization (WHO) proposed the integrated disease surveillance and response (IDSR) strategy to strengthen public health surveillance and response. In 2001, Uganda adapted the IDSR strategy and has since improved surveillance. An assessment conducted in 2007 to evaluate performance of the IDSR since its adaptation showed improvements in performance including improved reporting at district level, an increase in timeliness of reporting from district and central levels, an increase in analysed data, the case fatality rate for cholera and meningitis (targeted disease) reduced due to improved response and increased funding for IDSR (1). Another assessment conducted in 2016 additionally highlighted improvements of 69 to 100% in completeness of monthly reporting; monthly reporting from 59 to 78%, weekly from 40 t0 68%. Additionally the case fatality rate for cholera had reduced from 3,2% in 2012 to 2,1% in 2016 (2).
Context and importance of the problem
There is value in innovating early warning systems. In 2012, Uganda piloted a malaria monitoring system in Kabale and Rukingiri districts which automatically generated and analyzed malaria related data on a weekly basis. Electronic reports were disseminated by email to the National Control Programme. This monitoring system detected two malaria outbreaks in Kabale(3). Public health interventions were immediately mounted to respond to the malaria surge.
There is late detection of outbreaks using the current surveillance system which in turn leads to delayed response to public health emergencies. Although high pathogen diseases like Ebola Virus disease (EVD) can easily be detected due to their virulent nurture, other diseases of outbreak potential like malaria, typhoid, diarrhoea, and others might go unnoticed until the health system is overwhelmed.
Delayed detection of outbreaks results in increased transmission of diseases within communities and health care facilities. Although there is improved reporting by health facilities at district level, there is no automated mechanism to quickly analyse trends of diseases against the expected number of cases or threshold in a specific time period, immediately detect surges, and provide reports for verification and action. The manual approach to analysis of surveillance data is time consuming and cannot provide small unit (district/ sub county/ health facility) detailed trend analysis to inform quick decisions.
An automated mechanism to analyse all surveillance data collected from health facilities in the districts is critical for early detection, response, and containment of outbreaks at source with minimal transmission and deaths.
Critique of policy options
The World Health Organization through the International Health Regulations (IHR) recommends strengthening capacities to detect, assess, notify, and report events (4). This requires a surveillance system in place to collect information which Uganda has already developed. Additionally, the regulation requires the country to have prompt mechanisms to quickly detect surges or disease outbreaks which is still weak due to the slow analysis of all surveillance data.
Policy recommendations
The Ministry of Health should invest in creating a monitoring system or automated mechanism to quickly analyse all the data collected from the surveillance system and promptly create reports that are used for informed decision making and public health actions
References
- (PDF) The implementation of Integrated Disease Surveillance and Response in Uganda: A review of progress and challenges between 2001 and 2007 [Internet]. [cited 2020 Nov 27]. Available from: https://www.researchgate.net/publication/225273305_The_implementation_of_Integrated_Disease_Surveillance_and_Response_in_Uganda_A_review_of_progress_and_challenges_between_2001_and_2007
- Masiira B, Nakiire L, Kihembo C, Katushabe E, Natseri N, Nabukenya I, et al. Evaluation of integrated disease surveillance and response (IDSR) core and support functions after the revitalisation of IDSR in Uganda from 2012 to 2016. BMC Public Health. 2019 Jan 9;19(1):46.
- Cox J, Abeku T, Beard J, Turyeimuka J, Tumwesigye E, Okia M, et al. Detecting Epidemic Malaria, Uganda. Emerg Infect Dis. 2007 May;13(5):779–80.
- International Health Regulations, 2005 [Internet]. [cited 2020 Aug 2]. Available from: https://www.who.int/ihr/finalversion9Nov07.pdf
Transitioning to International Classification of Diseases (ICD)-11: The new, automated and user-friendly coding system to track diagnoses and procedures: A Policy Brief
Authors: Irene B. Kyamwine*1, Benon Kwesiga1, Alex Riolexus Ario11
1Uganda Public Health Fellowship Program, Kampala, Uganda
Executive summary
Uganda registration of persons act 2015 recommends that every birth and death be registered by civil registration. To achieve this Uganda adapted the manual system of International Classification of Diseases (ICD) version 10. Using this system, civil registration of death in 2016 was at only 24% compared to the set target 80% of deaths reported, registered, medically certified, and disaggregated by age and sex. The system is not flexible and is also expensive to implement hence it was not fully rolled out throughout the country. Recommending a transition from ICD-10 to ICD-11 and including ICD training in medical practitioners’ curricula would ease medical certification of cause of death, coding and hence civil registration coverage throughout the country.
Introduction
Uganda in the 1995 constitution recognized the need for registration of every birth, death, and marriage occurring throughout the country (1). The civil registration policy, 2012 recommended digitization of the registration process to enable improved or even complete birth and death registration (1,2). This policy also recognizes the importance of working together with other ministries/stakeholders such as Ministry of Health to achieve the target of registering all births and deaths. The National identification and Registration Authority (NIRA) under Ministry of Internal affairs is mandated under the Registration of Persons Act (ROPA) 2015 to register all births and deaths. Uganda adopted the use of International Classification of Diseases (1CD) 10 as a measure to streamline registration of both birth and deaths within the country. International Classification of Diseases (ICD) developed by World Health Organization is the international diagnostic classification standard for reporting diseases and health conditions globally. This allows for a common global language reporting, analysis, interpretation, and comparison of mortality and morbidity data (3,4). ICD can therefore be used for monitoring incidence and prevalence of diseases, reasons for encounter, factors that influence health status, and external causes of disease, counting of deaths, observing reimbursements, and resource allocation trends among others (4).
ICD undergoes revision to incorporate changes and updates in the practice of medicine. Since its inception in the 1940’s ICD has undergone several revisions to ICD-10 in 1990 and the current version ICD-11 in 2018 (5–7).
Context and importance of the problem
Registration of Persons Act (ROPA) 2015 mandates that registration of every death within Uganda is compulsory and a medical certificate of cause of death (MCCOD) must be issued (2). However, in 2016 only 24% of the deaths that occurred were registered by civil authority NIRA (8,9). This leaves a gap in the registration process in the zeal to implement the ROPA and Uganda draft civil registration Policy 2012.
To enable the implementation of this policy, there is need for a standardized and functional ICD system and a trained up to date health workforce that is able to accurately and timely code all the deaths as they occur using the ICD codes. Uganda, currently uses ICD-10 in health facilities to code death from MCCOD that are completed by medical practitioners (8). Given the limitations of ICD 10 as shown in Table 1, there is need to quickly transition to the revised ICD-11 in order to improve civil registration coverage.
Table 1: Comparison between International classification of diseases version-10 and 11
| ICD-10 | ICD-11 |
| 1) Manual curation.
a) Translation, updating and dissemination done manually b) Terminological inconsistency and poor quality translations c) Time-consuming implementation of updates. |
Automated
a) Search engine is customized for better and easier search results time saving b) Thousands of synonyms with global substitutions allowing terminological consistency c) Platform allows suggestions or additions to ICD–11 which are viewed and discussed transparently ensuring internationally consistent translations and the addition of locally used terms. |
| 2) Disseminated as a book
a) difficult & delayed integration of ICD in electronic health record systems and other software b) Expensive ICD-10 Books |
It is digital health ready, for use in multiple Information Technology (IT) environments |
| 3) Loss of international data comparability as users can not create their own shortlist and update list
|
In built guidance for use with different cultures and translations into 43 languages providing a common coding language. |
| 4) Quality of coded data compromised by coding errors despite expensive expert coder training
|
a) Requires less training hence improved ease and accuracy of coding
b) Includes an implementation package with components that ease the transition and better use the categorization system:5 |
| 5) Poor uptake & implementation of ICD
a) Lack of accurate disease information in countries with highest disease burden b) Delayed implementation
|
a) Thousands of synonyms with global substitutions
b) Reference guide text has been formatted using easier wordings to enhance user understanding
|
Critique of policy options
The currently used international classification system ICD-10, lacks automation functionality and thus requires translations, updating and dissemination to be done manually by individuals consequently leading to terminological inconsistency, poor quality translations, and time-consuming implementation of updates.
In addition, ICD-10 is disseminated as a book which causes difficult & delayed integration of ICD in electronic health record systems and other software, and Uganda being a low income country cannot afford to purchase ICD Books which leads to creation of shortlists and update lists not internationally understood hence loss of international data comparability.
Furthermore, the use of ICD-10 in the health care setting in Uganda is the role of the same health workers whose roles still continue regardless of the requirement to work as coders. The transition to ICD-10 was overly burdensome on providers who are already engaged in provision of other essential services since the government employment structure does not allow for employment of medical coders. Medical coders are health information professionals whose main duties are to analyze clinical statements and assign standard codes using a classification system in this case ICD-11. Given its manual nature, many facilities that implement ICD-10 leave the work to records officers who in some cases are not trained in ICD and do not have medical expertise to correctly code the cause of death from clinical notes. This exposes the country to inaccurate and inconsistent data (10).
Lastly, ICD has not been incorporated in any medical curriculum in the country which leads to a gap in the knowledge among Medical practitioners who are required by regulation to complete the NIRA cause of death form which deaths are coded according to ICD-10 format.
Recommendations
As Uganda looks forward to improved civil registration coverage as a way of attaining the Sustainable development goals, there is need to transition to a more robust system, ICD-11, that would allow for accurate and timely data on both mortality and morbidity. ICD-11 can be incorporated into existing electronic health applications and information systems. It also, allows for the clinician to document all clinical details. ICD-11 lowers the costs for using ICD since it requires less training and less time for coding, and as such allows the implementation of standard reporting (11).
In addition, medical practitioners’ curriculum should be revised to include training on international classification of diseases (ICD-11) to allow for accurate and consistent reporting of morbidity and mortality. Also, there is need to put in place capacity building strategies to enlighten health workers that are already in practice on ICD-11.
Finally, the government needs to revise the employment structure to allow for other cadres such as coders to help boost the capacity of health facilities with skilled human resource to accurately provide this very relevant information for civil registration.
Reference
- van der Straaten J. Uganda Draft Civil Registration Policy 2012. 2012.
- REGISTRATION OF PERSONS ACT , 2015 | Uganda Legal Information Institute [Internet]. [cited 2020 Oct 10]. Available from: https://ulii.org/ug/legislation/act/2015/4-6
- ICD-11 Implementation or Transition Guide_v105.pdf [Internet]. [cited 2020 Oct 10]. Available from: https://icd.who.int/docs/ICD-11%20Implementation%20or%20Transition%20Guide_v105.pdf
- WHO | International Classification of Diseases, 11th Revision (ICD-11) [Internet]. WHO. World Health Organization; [cited 2020 Oct 10]. Available from: http://www.who.int/classifications/icd/en/
- reportoftheicd11review14april2015.pdf [Internet]. [cited 2020 Oct 10]. Available from: https://www.who.int/classifications/icd/reportoftheicd11review14april2015.pdf?ua=1
- International Classification of Diseases [Internet]. [cited 2020 Oct 10]. Available from: https://www.who.int/news-room/spotlight/international-classification-of-diseases
- WHO releases new International Classification of Diseases (ICD 11) [Internet]. [cited 2020 Oct 10]. Available from: https://www.who.int/news-room/detail/18-06-2018-who-releases-new-international-classification-of-diseases-(icd-11)
- Snapshot of civil registration and vital statistics systems of Uganda. :16.
- Government of Uganda, Uganda Bureau of Statistics, The DHS Program ICF. Uganda Demographic and Health Survey 2016. 2018.
- Jette N, Quan H, Hemmelgarn B, Drösler S, Maass C, Moskal L, et al. The Development, Evolution, and Modifications of ICD-10: Challenges to the International Comparability of Morbidity Data. Med Care. 2010 Oct 1;48:1105–10.
- icd11factsheet_en.pdf [Internet]. [cited 2020 Oct 10]. Available from: https://icd.who.int/en/docs/icd11factsheet_en.pdf
Improving Malaria Reporting by Village Health Teams under Integrated Community Case Management:
A Policy Brief:
Authors: Gerald B. Rukundo*1, Benon Kwesiga1, Alex Riolexus Ario1
1Uganda Public Health Fellowship Program, Kampala, Uganda
Executive Summary
Malaria remains among the leading causes of morbidity and mortality in children under 5 years of age. To address this gap, in 2010 Uganda adopted the Integrated Community Case Management (iCCM) strategy to promote community level management of malaria among children under 5 years of age. This ICCM strategy is implemented by Village health teams (VHTs) who record and report malaria cases treated. Presently, recording and reporting malaria cases is paper based and this has presented many challenges. The use of paper brings unnecessary data incompleteness & delays in reporting. Poor data recording practices by VHTs and lack of supervision, often affect data quality. Unlike health facilities which report on a weekly and monthly basis, VHTs report on a quarterly basis. This often leads to under-utilization of malaria surveillance data generated by VHTs. To improve on reporting and data utilization, there is a need to harmonize the frequency of reporting to the DHIS2 by both VHTs and health facilities. There is a need by ministry of health to pilot digital reporting to improve on data quality and timeliness. Mobile reporting system could reduce the time required for malaria treated cases to be reported by the VHTs to the district, and national levels. The mobile reporting system is a feasible option to assist with early detection of malaria outbreaks.
Background
Uganda is a malaria endemic country with active transmission in 99% of the country putting approximately 39 million people at risk [1]. The most vulnerable populations are pregnant women and children under 5 years of age. According to the 2016 Uganda demographic and health survey (UDHS), the malaria prevalence among children under 5 years of age by rapid diagnostic test (RDT) was at 30% [1]. The 2018-2019 malaria indicator survey found the malaria prevalence of children under 5 at 17%.
Malaria remains among the leading causes of morbidity and mortality in children under 5 years of age [2,3]. To address this gap, in 2010 Uganda with support from United Nations Children’s Fund (UNICEF), adopted the Integrated Community Case Management (iCCM) strategy to promote community level management of malaria among children under 5. The iCCM strategy was initially rolled out in 22 districts but later expanded to other districts, most especially in areas considered to be hard to reach with limited access to health care.
Currently the iCCM strategy is being implemented by village health teams (VHTs) and has demonstrated that the use of VHTs expands malaria treatment areas hence resulting into reduction of malaria morbidity and mortality [4,5]. Under this strategy, VHTs are usually given health information management (HMIS) tools to record and report malaria cases treated. The current reporting rate by VHTs to the national health information system remains low compared to health facility reporting.
Several studies have demonstrated the ability of VHTs to collect epidemiological data on a variety of diseases, including malaria [6-12]. In poorly-resourced countries, community-based surveillance systems are best suited to complement health facility (HF)-based surveillance. Community-based surveillance systems provide quantitative estimates of disease burden in a defined population and service delivery indicators for disease control measures [16] but remain under-exploited in relation to malaria.
Public health surveillance, has been defined as the “ongoing systematic collection, analysis, and interpretation of data critical to the planning, implementation, and evaluation of public health interventions” [17,18]. Effective use of such surveillance data requires timely dissemination to all relevant stakeholders [17,18]. Effective systems for detecting and reporting malaria infection in human populations have an increasingly important role to play as control steadily progresses towards elimination so that infection and disease become more focal in time and space and additional interventions are increasingly targeted in response to surveillance data [19,20].
As we move towards malaria elimination, reporting malaria cases becomes increasingly critical, to halt continuing transmission.
Currently, there are two categories of VHTs that report malaria cases. These include VHTs who work under the Ministry of Health (MOH) system and partners. The VHTs under MOH are volunteers who use paper to report malaria cases to the nearest health facility on a quarterly basis. The health facility then reports directly to the district where data is entered into the district health information system (DHIS2). Partner VHTs are supported by donors & small programs. They move door to door selling medical products and receive incentives depending on the number of medical products sold. They report directly to the donor platforms using mobile technology. Presently, there are challenges in the reporting systems used by the VHTs to report malaria cases.
Importance of the problem
The current HMIS guidelines allows health facilities to report weekly, monthly, and quarterly malaria cases to the DHIS2 and yet VHTs report on quarterly basis. Due to the difference in frequency of reporting, data generated by the VHTs is never utilized while responding to malaria outbreaks. The contribution of VHTs to malaria control in Uganda is usually underestimated and yet they treat a considerable high number of children under 5. To effectively manage malaria epidemics and move towards elimination, timely provision of accurate malaria surveillance data is necessary [21].
The completeness, accuracy, and timeliness of HMIS used by VHTs is often inadequate. These systematic weaknesses undermine stakeholder confidence in the reliability of this data and, consequently lead to its under-utilization for decision-making and planning [22]. Data quality is usually compromised due to multiple reporting forms, registers, and reporting levels. Most times VHTs fail to report malaria cases upwards due to many factors such as lack of transport to deliver reports to the facility, stock out of reporting tools, and inability to comprehend reporting tools.
To control and eventually eliminate malaria from Uganda there is a need to detect, treat, and notify cases in a timely way. Strengthening the malaria surveillance system in Uganda will allow more efficient and targeted allocation of resources to help interrupt transmission and achieve total malaria.
Critique of current policy options
Presently, the national malaria control program uses paper-based reporting system, whereby VHTs perform malaria testing and record the individual’s information on a paper form. Data is then aggregated at the end of every quarter and has to pass through multiple reporting levels to reach the District health information system (DHIS2). Use of paper by VHTs in reporting presents many challenges. Use of paper brings unnecessary data incompleteness & delays in reporting. Poor data recording practices and lack of supervision, affect surveillance data quality. In some settings, failure of VHTs to completely report upwards in the reporting chain has resulted into aggregation of incomplete datasets and generalized under-reporting of malaria burden in communities. Most times, reporting is affected by lack of transport, motivation, and poor terrain. Additionally, the guideline of VHTs reporting to higher levels of the health system on a quarterly basis is slow for any rapid action. The use of weekly malaria surveillance data to quickly identify malaria outbreaks leaves data generated by VHTs on quarterly basis redundant. Due to slow reporting by VHTs, data are never utilized by epidemiologists. Additionally, multiple reporting forms and registers used by the VHTs coupled with multiple reporting levels compromise data quality.
In contrast VHTs supported by partners use mobile phone-based application tool, which allows to report malaria testing results on-the-spot, with the aim of allowing stakeholders’ access to up-to-date data in real-time. Additionally, the VHTs supported by partners’ report to an independent platform. The MOH and partner VHT reporting systems don’t interact and therefore data is never aggregated. Failure to integrate the two reporting systems gives a wrong impression on the actual number of malaria cases treated by VHTs and may lead to under-estimation of the malaria burden in the country.
Recommendations
The frequency of reporting to the DHIS2 by both VHTs and health facilities need to be aligned to effectively identify the true malaria burden at any given moment. To improve on data quality and timeliness, multiple reporting tools and levels need to be eliminated. Like partners, MOH needs to pilot digital reporting to avoid unnecessary delays and improve data quality. Mobile reporting system reduces the time required for diagnosed cases to be reported by the health care facility to district, and national levels. The mobile reporting system is a feasible option to assist with early detection of malaria outbreaks. To minimize over reporting of malaria cases, there is a need to integrate the two parallel VHT reporting systems (under MOH & partners). Data generated by VHTs needs to be utilized by epidemiologists and public health planners.
References
- Uganda malaria Annual report 2017/2018
- Marsh DR, Hamer DH, Pagnoni F, Peterson S. Introduction to a special supplement: evidence for the implementation, effects, and impact of the integrated community case management strategy to treat childhood infection. Am J Trop MedHyg. 2012;87:2-5.
- World Health Organization. WHO/UNICEF joint statement integrated community case management (iCCM): an equity-focused strategy to improve access to essential treatment services for children. Geneva and New York; 2012.
- Lemma H, Byass P, Desta A, et al (2010) Deploying artemether-lumefantrine with rapid testing in Ethiopian communities: impact on malaria morbidity, mortality and healthcare resources. Tropical Medicine and International Health 15, 241–250
- Kamal-Yanni MM, Potet J, Saunders P (2012) Scaling-up malaria treatment: a review of the performance of different providers. Malaria Journal 11, 414.
- Hamainza B, Moonga H, Sikaala C, Kamuliwo M, Bennett A, Eisele T, Miller J, Seyoum A, Killeen G. Monitoring, characterization and control of chronic, symptomatic malaria infections in rural Zambia through monthly household visits by paid community health workers. Malar J. 2014;13:128. doi: 10.1186/1475-2875-13-128.
- Counihan H, Harvey SA, Sekeseke-Chinyama M, Hamainza B, Banda R, Malambo T, Masaninga F, Bell D. Community health workers use malaria rapid diagnostic tests (RDTs) safely and accurately: results of a longitudinal study in Zambia. Am J Trop Med Hyg. 2012;87:57–63. doi: 10.4269/ajtmh.2012.11-0800
- Kalyango JN, Rutebemberwa E, Alfven T, Ssali S, Peterson S, Karamagi C. Performance of community health workers under integrated community case management of childhood illnesses in eastern Uganda. Malar J. 2012;11:282. doi: 10.1186/1475-2875-11-282.
- Alba S, Hetzel MW, Nathan R, Alexander M, Lengeler C. Assessing the impact of malaria interventions on morbidity through a community-based surveillance system. Int J Epidemiol. 2011;40:405–416. doi: 10.1093/ije/dyq240. [PubMed] [CrossRef] [Google Scholar]
- Hopkins H, Talisuna A, Whitty CJ, Staedke SG. Impact of home-based management of malaria on health outcomes in Africa: a systematic review of the evidence. Malar J. 2007;6:134. doi: 10.1186/1475-2875-6-134.
- Rutta AS, Francis F, Mmbando B, Ishengoma D, Sembuche S, Malecela E, Sadi J, Kamugisha M, Lemnge M. Using community-owned resource persons to provide early diagnosis and treatment and estimate malaria burden at community level in north-eastern Tanzania. Malar J. 2012;11:152. doi: 10.1186/1475-2875-11-152.
- WHO. Disease Surveillance for Malaria Elimination: An Operational Manual. Geneva: World Health Organization; 2012. [
- Chanda P, Hamainza B, Moonga HB, Chalwe V, Pagnoni F. Community case management of malaria using ACT and RDT in two districts in Zambia: achieving high adherence to test results using community health workers. Malar J. 2011;10:158. doi: 10.1186/1475-2875-10-158.
- Ruebush TK, II, Godoy HA. Community participation in malaria surveillance and treatment.
I5. The Volunteer Collaborator Network of Guatemala. Am J Trop Med Hyg. 1992;46:248–260.
- Oum S, Chandramohan D, Cairncross S. Community-based surveillance: a pilot study from rural Cambodia. Trop Med Int Health. 2005;10:689–697. doi: 10.1111/j.1365-3156.2005.01445.x
- Thacker SB, Berkelman RL. Public health surveillance in the United States. Epidemiol Rev. 1988;10:164–190.
- WHO. Manual on Epidemiology Evaluation and Surveillance in Malaria Eradication. Geneva: World Health Organisation; 1962.
- Wetterhall SF, Pappaioanou M, Thacker SB, Eaker E, Churchill RE. The role of public health surveillance: information for effective action in public health. MMWR Morb Mortal Wkly Rep. 1992;41(Suppl):207–218.
- Barclay VC, Smith RA, Findeis JL. Surveillance considerations for malaria elimination. Malar J. 2012;11:304. doi: 10.1186/1475-2875-11-304.
Baker EL, Jr, Ross D. Information and surveillance systems and community health: building the public health information infrastructure. J Public Health Manag Pract. 1996;2:58–60. doi: 10.1097/00124784-199623000-00016.
22. de Savigny D, Binka F. Monitoring future impact on malaria burden in sub-saharan Africa. Am J Trop Med Hyg. 2004;71:224–231.
Uptake of Human Papilloma Vaccination in Uganda, Barriers and Opportunities: A Policy Brief
Author: Dr. John Kamulegeya, Uganda Public Health Fellowship Program, Kampala, Uganda
Summary
Human papillomavirus (HPV) infection is the primary cause of cervical cancer. Cervical cancer is the leading cause of cancer deaths among women in Uganda and Sub Saharan Africa. HPV types 16 and 18 are responsible for about 70% of all cervical cancer cases worldwide. Vaccination against these prominent types of human papilloma virus has the potential to drastically reduce HPV-associated diseases, including cervical and other anogenital cancers. HPV vaccine against two sero types, 16 and 18 has been available for routine immunization since 2014, targeting 10 year old girls using a two-dose schedule with an interval of six months between doses. However, HPV vaccination uptake is low with less than 50% of targeted girls receiving their 2nd follow up dose. A number of individual, community, and health system factors affect HPV vaccination. Multi prong strategies aimed at reaching younger girls, empowering the girl child and their parents /caretakers to demand for HPV vaccination services should be embraced in order to achieve high vaccination uptake in Uganda.
The policy brief is intended to strengthen the guidance for health policy implementation of HPV vaccination in Uganda.
Background
Human papillomavirus (HPV), is a highly prevalent sexually trans-mitted infection. Human papillomavirus is the primary cause of cervical cancer (>99% of cases) (1). Vaccination against prominent types of human papillomavirus (HPV) has the potential to dramatically reduce HPV-associated diseases, including cancer. However vaccine uptake has been variable and suboptimal in most countries, with low levels of both initiation and completion of the HPV doses (2). A woman’s lifetime risk of acquiring HPV infection is greater than 80% and most infections occur within 3–4 years of sexual debut. Among HIV positive women, the prevalence of HPV infections and high grade cervical pre-cancer lesions is several folds higher than in HIV negative women.
Approximately 90% of deaths from cervical cancer occur in low- and middle-income countries. The high mortality rate from cervical cancer globally could be reduced through a comprehensive approach that includes prevention through HPV vaccination, early diagnosis, effective screening and treatment programmes. Women in low- as opposed to those in high-income settings have about a twofold cumulative risk of developing cervical cancer before the age of 65 years. Equally, women in low income settings have a threefold risk of dying from cervical cancer than those in high income settings(3, 4).
In developing countries, primary prevention through Human papilloma vaccination is the most feasible and cost effective method to reduce the morbidity and mortality due to CxCa . With moderate financial support, it is feasible to archive high vaccination rates by using existing education and health infrastructure(5). An optimal coverage (≥70%) of the target population, the lifetime risk of cervical cancer could be reduced by >50%.
Oncogenic HPV types 16 and 18 are responsible for over 70% of cervical cancers, globally. Uganda has one of the highest cervical cancer incidence rates in the world with the age-standardized incidence rate of 47.5 per 100, 000. Cervical cancer(CxCa) is the leading cancer among women in Uganda , contributing up to about 50–60% of all female malignancies(6) .
In Uganda, preventive HPV vaccine that protect against two sero types HPV 16 and 18 has been available for routine immunisation since 2014, using a two-dose schedule with an interval of six months between doses for human papillomavirus(HPV) vaccine.
Ugandan targets to vaccinate all girls aged 10 years. The strategy used is reaching out to pupils in primary four. Vaccination is delivered during every static and outreach immunization sessions but intensified through Integrated Child Health Days during the months of April and October where health workers conduct school-based outreaches.
We explored uptake of HPV vaccination, implementation challenges (cultural barriers, operational and logistical) so to identify and recommend strategic interventions to improve HPV vaccination uptake in Uganda.
Problem Analysis
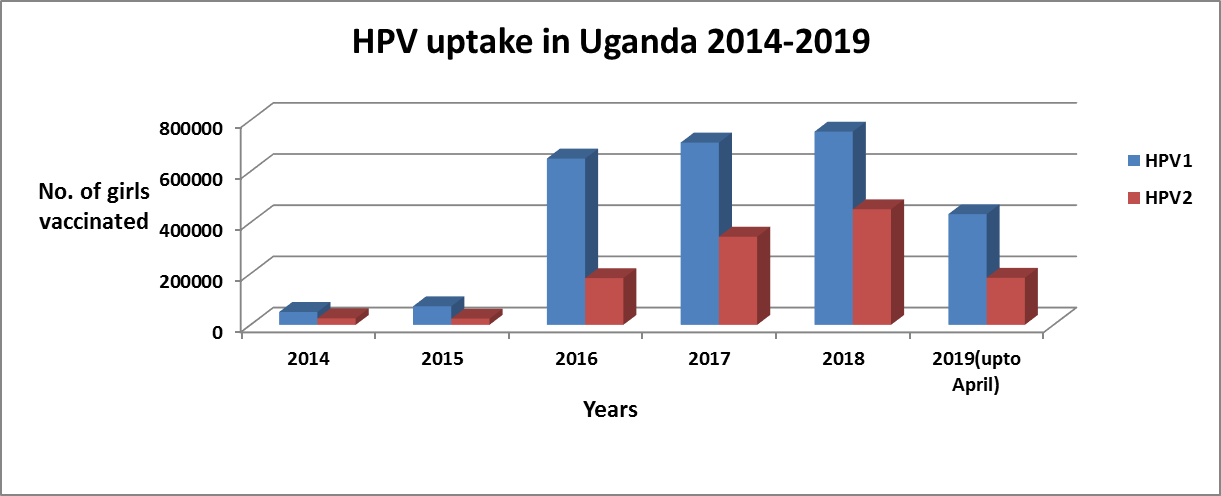
Despite HPV vaccination being free, HPV vaccination uptake is low with less than 50% of targeted girls receiving their 2nd follow up dose. In addition, the routine immunisation program is not reaching out to all targeted girls (Figure 1) (Source-District Health Information System-DHIS 2)
Factors affecting uptake of Human Papilloma Vaccination, Uganda
HPV vaccine uptake (i.e., initiation and completion) among eligible girls is affected by a number of factors. These include, health system factors, individual factors such as HPV awareness and knowledge, beliefs about HPV and the vaccine (e.g., perceived vaccine effectiveness, daughters ‘perceived risk for HPV, perceived severity of HPV infection), general immunization attitudes and demographics of parents. Cultural and religious factors have been shown to affect immunization uptake
Human papillomavirus (HPV) vaccine uptake in many countries has been sub-optimal. Several individual factors are associated with low vaccination including fears about sexual risk , concerns about vaccine safety, inadequate vaccination recommendations by health care providers (HCPs), and distrust due to the perceived “newness” of HPV vaccines(7).
HPV vaccination presents special challenge for the fact that it targets sexually transmitted disease yet given to adolescent girls who have not yet had sexual intercourse. Secondly, the primary efficacy endpoint was a surrogate for invasive cancer, which takes decades to develop after initial HPV infection. It is because of this that some communities in Uganda claim that the vaccine has a contraceptive component that can cause infertility.
Teachers’ knowledge
Since one of the major delivery strategies is through school outreaches, it critical that teachers to the pupils have adequate knowledge on HPV vaccination and cervical cancer. Studies have found out that knowledge about HPV vaccine among school teachers was low. Empowering teachers to be vaccine champions in their community may be a feasible way of disseminating information about HPV vaccine and cervical cancer. Teachers with little knowledge on HPV vaccine are less likely to accept the vaccine than those who know more(8).
Health system factors
Health service delivery challenges are one of the greatest barriers to HPV vaccination, specifically the lack of capacity to track and distribute reminders to eligible patients, low ability to effectively mobilise communities and commodity logistic challenges. Also variation in health workers’ counseling approaches to emphasize cancer prevention benefits of the vaccine has been noted as barriers to HPV uptake (9, 10). Health care providers do not strongly recommend the vaccine, are not effectively educating their patients about it, are not outlining its vaccination schedule, and are not urging families to start and then complete the vaccination in time.
Community/societal factors
In addition, parental consent for daughters to receive the HPV vaccination is critical for the HPV vaccination programme (11). Engagement of all stakeholders is critical for successful HPV vaccination. Leadership by public health professionals and clinicians and nurses is essential but parents, teachers, schools, legislators, religious and cultural leaders, all need to be involved at both planning and implementation of HPV vaccination in communities (12).
Because of culture, adults feel embarrassed talking about sex with “children,” This is exacerbated by the fact that different cultures have strongly held taboos about children sex education this process. Unlike other vaccine preventable diseases such as polio, measles, pneumonia and meningitis, which most parents have at least heard of and know enough to fear, HPV is a largely unknown entity(13).
Solution Analysis
The success of future human papillomavirus (HPV) vaccination programs will depend on individuals’ willingness to accept vaccination, parents’ willingness to have their preadolescent and early adolescent children vaccinated, and health care providers’ willingness to recommend HPV vaccination(14).
Involvement of Adolescents, Care takers, and Parents
Studies have found that individuals’ knowledge and attitudes toward the vaccine are associated with immunization uptake. Providing substantial information for participants, particularly adolescents who may exercise a significant level of autonomy in decision-making can greatly increase HPV vaccination uptake
Research to understand reasons for low uptake
Since HPV vaccine is administered prior to an adolescent’s sexual debut, this ultimately requires understanding decision-making for a young adolescent to receive a vaccine targeting a sexually transmitted infection (STI). Given the fact that vaccinating pre-adolescents and adolescents is a relatively new phenomenon in many resource-limited settings, formative socio-behavioral research is essential for providing a framework for optimizing vaccine uptake(14). Qualitative research to understand psychosocial barriers to HPV vaccination, including concerns about vaccine safety and efficacy, and its impact on future fertility in order to effectively design programs that would optimize vaccine uptake.
Use of care givers and peer and cultural influencers
In a study in South Africa on uptake of HPV vaccines, caregivers expressed difficulty initiating conversations with their adolescents related to sexual activity or sexual risk-taking, due to lack of information about HPV as well as cultural constrains. Healthcare providers at the adolescent clinic were described as adult proxies who could have conversations with their children about sexual health, in order to ‘‘provide knowledge about sexual issues
Parents value the information and recommendations provided by their children’s health care providers. At the same time, health care providers will need to be prepared to provide patients and parents with information about HPV and HPV immunization and to respond productively to the rare parent who expresses opposition to HPV vaccine(10).
Recommendations
Community Dialogue
Community dialogues have the potential to alleviate some critical concerns that parents may have when vaccinating their children against HPV. The dialogues should emphasize HPV vaccination benefits. Studies that have examined the dyadic process of vaccine decision-making between parents and adolescents have identified benefits that result from the process itself as well as the communications surrounding HPV vaccine. In addition, information given in community dialogues communication about HPV vaccine may improve parents discussions with young adolescents on positive sexual health. Community dialogues will bridge information and communication gap between parents, young adults, adolescents, and health care providers so that higher HPV vaccination rates can be achieved
Community sensitization
Accumulating evidence suggests that many of the social/behavioral concerns associated with HPV vaccine that have sparked resistance among patients and providers (and have been the focus of media reports) have little or no basis in reality (16). Community sensitization using mass media such as radio, TV and social media platforms to correct miss-information may improve uptake of HPV in Uganda.
Involvement of Teachers
Since the greatest target population for HPV vaccination is in primary schools, teachers should be given enough information about HPV vaccination, its importance and safety. In addition, teachers and schools’ administration should be involved in all stages of planning, monitoring and vaccination implementation.
Reminders and Brochures
Electronic alerts prompted telephone reminders for dose completion. These results show that an evidence-based educational brochure and reminder system appeared to improve HPV vaccine uptake and dose completion rates at this private pediatric practice. Electronic alerts prompted telephone reminders for dose completion(15)
Research to identify barriers and opportunities to improve HPV vaccination uptake
Research has shown that the reasons for and expressions of vaccine hesitancy are highly varied and need to be better understood in order to appropriately address emerging concerns. Reasons for suboptimal HPV vaccination coverage will be better understood by carrying out a qualitative and quantitative research in Uganda in order to inform policy.
References
- Munoz N. Human papillomavirus and cancer: the epidemiological evidence. Journal of clinical virology. 2000;19(1):1-5.
- Gattoc L, Nair N, Ault K. Human papillomavirus vaccination: current indications and future directions. Obstet Gynecol Clin North Am. 2013;40(2):177-97.
- Shanta V, Krishnamurthi S, Gajalakshmi C, Swaminathan R, Ravichandran K. Epidemiology of cancer of the cervix: global and national perspective. Journal of the Indian Medical association. 2000;98(2):49.
- WHO. Comprehensive cervical cancer control, A guide to essential practice. 2006.
- Goldie SJ, O’Shea M, Campos NG, Diaz M, Sweet S, Kim S-Y. Health and economic outcomes of HPV 16,18 vaccination in 72 GAVI-eligible countries. Vaccine. 2008;26(32):4080-93.
- Wabinga H, Parkin D, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960-1997. British Journal of Cancer. 2000;82(9):1585-92.
- Zimet GD, Rosberger Z, Fisher WA, Perez S, Stupiansky NW. Beliefs, behaviors and HPV vaccine: Correcting the myths and the misinformation. Preventive Medicine. 2013;57(5):414-8.
- Mabeya H, Menon S, Weyers S, Naanyu V, Mwaliko E, Kirop E, et al. Uptake of three doses of HPV vaccine by primary school girls in Eldoret, Kenya; a prospective cohort study in a malaria endemic setting. BMC Cancer. 2018;18(1):557.
- Sussman AL, Helitzer D, Bennett A, Solares A, Lanoue M, Getrich CM. Catching Up With the HPV Vaccine: Challenges and Opportunities in Primary Care. The Annals of Family Medicine. 2015;13(4):354-60.
- Zimet GD. Improving adolescent health: Focus on HPV vaccine acceptance. Journal of Adolescent Health. 2005;37(6, Supplement):S17-S23.
- Buang SN, Ja’afar S, Pathmanathan I, Saint V. Human papillomavirus immunisation of adolescent girls: improving coverage through multisectoral collaboration in Malaysia. BMJ. 2018;363:k4602.
- Yildirim JG, Arabaci Z. Innovations in HPV vaccination and roles of nurses in cervical cancer prevention. Asian Pacific journal of cancer prevention : APJCP. 2014;15(23):10053-6.
- Palfrey S. New initiatives to improve HPV vaccination rates. Human Vaccines & Immunotherapeutics. 2016;12(6):1594-8.
- Katz IT, Nkala B, Dietrich J, Wallace M, Bekker L-G, Pollenz K, et al. A Qualitative Analysis of Factors Influencing HPV Vaccine Uptake in Soweto, South Africa among Adolescents and Their Caregivers. PLoS One. 2013;8(8):e72094.
- Cassidy B, Braxter B, Charron-Prochownik D, Schlenk EA. A Quality Improvement Initiative to Increase HPV Vaccine Rates Using an Educational and Reminder Strategy With Parents of Preteen Girls. Journal of Pediatric Health Care. 2014;28(2):155-64.
- Zimet GD, Rosberger Z, Fisher WA, Perez S, Stupiansky NW. Beliefs, behaviors and HPV vaccine: correcting the myths and the misinformation. Prev Med. 2013;57(5):414-8.
The Uganda Public Health Fellowship Program Trains District Health Teams in five Districts of Lango on Early Detection of Malaria Outbreaks, September 2020
Authors: Job Morukileng1, Namayanja Josephine1 Alex Ndyabakira1 and Alex Riolexus Ario1 1Uganda Public Health Fellowship Program, Kampala, Uganda
Background
Malaria remains a major public health problem and the most frequently reported disease at both public and private health facilities in Uganda. The disease accounts for 30-50% of outpatient visits, 15-20% of admissions, and up to 20% of all facility deaths [1,2]. In 2019, there was a malaria upsurge in some of the districts in Uganda. These upsurges were detected late due to failure to analyze surveillance data both at the district and national level. The District Health Team (DHT) is responsible for monitoring malaria data collection, analysis, and reporting in the district. The World Health Organisation recommends plotting a malaria normal channel in malaria endemic countries including Uganda as a method for detecting outbreaks. Malaria normal channel refers to the normal seasonal pattern of malaria in an area [3], beyond which, an outbreak of malaria is detected. We trained DHT members on how to plot and interpret malaria normal channel graphs to facilitate early detection and response to malaria outbreaks.
Methods
We selected districts that registered upsurges of malaria case patients in 2019 (outbreak period) for immediate training on development of malaria normal channels. In Lango sub-region, we selected five districts including; Kole, Apac, Oyam, Alebtong, and Dokolo all located in in Northern Uganda.
We targeted a team of 50 participants, 10 participants per district, and the participant categories included: the DHO, malaria focal person, Health Management Information System (HMIS) focal person, District Biostatistician, District Surveillance Focal Person (DSFP), Assistant-DHO Maternal Child Health, Assistant-DHO Environmental Health, plus 3 other participants selected by the district (for example, representatives from main hospital or health sub-district).
We used a case study developed by Uganda Public Health Fellowship Program in 2019 (Analysis of malaria surveillance data (2014-2019) to train the DHTs to draw and interpret a malaria normal channel. The case study initial session recaps the participants on the general knowledge on surveillance, its importance, the different types of surveillance and advantages and disadvantages of each type, followed by the step by step hands on instructions on how to construct the malaria normal channel graphs.
We trained participants on two main methods of plotting malaria normal channels, that is, mean and two standard deviations (2SD) method and percentile method. Participants used computers/laptops and graph papers to construct malaria normal channels and were guided on how to interpret them.
Achievements
Of the targeted 50 participants, we trained 40 (80%) DHT members from five districts of Kole, Apac, Oyam, Alebtong, and Dokolo in five days, one day training per district (Table 2).
Table 2: Participant categories, the total number expected, and trained in the five districts
| Participant category | Expected | Trained | Proportion |
| District Health Officer (DHO) | 5 | 4 | 80 |
| Surveillance Focal Persons | 5 | 5 | 100 |
| Malaria Focal Persons | 5 | 5 | 100 |
| Ass. DHO Maternal Child Health | 5 | 5 | 100 |
| Ass. DHO Environmental Health | 5 | 5 | 100 |
| District Biostatistician | 5 | 5 | 100 |
| Hospital representatives | 5 | 0 | 0 |
| health sub-district representative | 10 | 6 | 60 |
| Health Management Information System Focal Person | 5 | 5 | 100 |
| Total | 50 | 40 | 80 |
Observations
Some of the districts had conflicting activities. The presence of other activities did not only lead to low turn up -, but also led to divided attention among those who participated. However, hands-on practice caught the attention of the participants because each of them was required to display graphs they had plotted. All the biostatisticians in the five districts were familiar with the mean +2SD method of developing malaria normal channels. However, following the training, they appreciated the fact that the percentile method is more sensitive in detecting the malaria outbreaks earlier than the mean method.
Conclusions and recommendations:
The training was well attended (80% of the participants turned up) and at the end of the training, each participant was practically able to plot a malaria normal channel graph and interpret it. We recommended that the trained DHT members should organise a session to orient those who missed the training. We also recommended that, malaria normal channels should be plotted during quarterly meetings and the graphs interpreted to facilitate early detection and guide actions.
Figure 1: Namayanja Josephine giving step by step instructions for development of malaria normal channels in Oyam District

Figure 2: Morukileng Job (fellow) taking participant through an over view of surveillance prior to introduction malaria normal channels development in Apac District

References
- Uganda – Malaria Operational Plan FY 2019. :82.
- World Malaria Report 2015. In: WHO [Internet]. 2015 [cited 26 May 2016]. http://www.who.int/malaria/publications/world-malaria-report-2015/en/ [Ref list]
- Prevention and control of malaria epidemics: Tutor’s Guide; WHO, 2003.
- Ssempiira J, Nambuusi B, Kissa J, Agaba B, Makumbi F, Kasasa S, et al. Geostatistical modelling of malaria indicator survey data to assess the effects of interventions on the geographical distribution of malaria prevalence in children less than 5 years in Uganda. PLoS ONE [Internet]. 2017 Apr 4 [cited 2020 Jan 28];12(4). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5380319/
- Muhaise H, Kareeyo DM. Electronic Health Information Systems Critical Implementation Issues (E-HMIS): District Health Information Software Version.2 in the Greater Bushenyi, Uganda. Am Sci Res J Eng Technol Sci ASRJETS. 2017 Aug 11;34(1):205–12.
Cost effectiveness and decision analysis for evaluation of the national airport screening options in COVID-19 surveillance in Uganda, 2020
Author: Geofrey Amanya N,
Uganda Public Health Fellowship Program, Kampala, Uganda
Executive Summary
Early during the COVID-19 outbreak, various approaches were utilized around the world to preventing introduction of COVID-19 from incoming airport travellers. However, the costs and effectiveness of airport-specific interventions had not been evaluated. We evaluated different policy options for COVID-19-specific interventions at Entebbe International Airport to inform decision-making in future similar situations. Screening all incoming travellers for symptoms, testing symptomatic persons, and isolating positives (Option 2) was the most cost-effective option for airport interventions against COVID-19. Higher prevalence of infection among incoming travellers increased cost-effectiveness of airport-specific interventions. This model may be used to evaluate prevention options at the airport for COVID-19 and other diseases with similar requirements for control.
Introduction
The increase in global air travel provides countless opportunities for infections to spread, both to passengers on the plane and also to the community after arrival (1). Since the advent of the SARS-CoV-2 pandemic, multiple interventions were employed in countries around the world to limit or slow the introduction of SARS-CoV-2 by air travellers from highly-affected areas. These approaches included obligatory quarantine policies for travellers, and border closures, and instituted local or national lockdowns (2). These strategies have varied across nations, with varied levels of sustainability, consideration of the resources of health care systems, and acceptability to the community (3). While no country has succeeded in maintaining an entirely COVID-19-free state, interventions aimed at travellers (2) almost certainly managed to delay or reduce the impact of the epidemic in multiple countries (3, 5).
Uganda has a single large international airport (Entebbe International Airport (EBB)) through which the vast majority of international air travel occurs in the country. Although its three smaller domestic airports do receive a few short-range flights from nearby neighbouring countries, 63% of incoming international travellers entering Uganda enter through EBB.
Since the early case of COVID was reported in Uganda on February 2020, a number of control measures were implemented at the airport such as IPC, installed hand sanitizer stations, queue separators to keep people from crowding while they waited. In addition, the MoH did risk mitigation by mapping out travellers from high at-risk countries, this managed to reduce and contain the disease, however with unlimited traveling through other points of entries, the cases kept increasing.
COVID-19 will cease to be a travel-associated threat once vaccines become widely available and used. However, future epidemics during which airport-specific interventions will again become relevant are all but certain: screening for Ebola Virus Disease was still in place at EBB when COVID-19 screening began in February 2020. Despite this, there have been relatively few studies on the cost-effectiveness of preventive interventions for COVID-19. We compared the cost-effectiveness of different policy options for COVID-19-specific interventions at EBB to guide decision-making by national stakeholders during this and future epidemics.
Context and importance of the problem
Policy Option 1: No intervention. Under this policy, there is no screening at the airport and no quarantine or isolation associated with persons entering at the airport. Incoming travellers may require isolation or quarantine, but not through any program affiliated with the airport. This is what would occur in the absence of a public health threat, where a proportion of incoming travellers might be ill and seek diagnosis or treatment at their own expense.
Policy Option 3: Mandatory quarantine for all, symptom screening testing for all, and Isolating the positives. Under this policy, all incoming travellers would undergo institutional quarantine for 14 days, there would be close symptom monitoring/follow up then testing would be done, if a person turns out positive, they would be transferred to an isolation unit.
Outcomes
The primary outcome measured was the incremental cost-effectiveness ratio (ICER) per COVID-19 case prevented. Secondary outcomes included expected value per incoming traveller at EBB, cases identified through the airport programme, cases that end up in the community, case counts after a single generation of cases from infected travellers entering the community, and the average costs per estimated traveller for each policy option.
Methods
We used a multiple criteria decision analysis to compare three different airport interventions for costs and impact on case counts over a two-week time horizon, with the primary outcome of cost per case averted. We took the government perspective.
Results
At a prevalence of 5% for COVID-19 among the incoming travellers, a total of 3,375, cases go through airport (for each of the option), a total of 1,369 will be identified by policy option 2, and 2,363 for policy option 3, a total of 12,150 1st generation cases will be identified for option 1; 5,722 for option 2 and 3,195 for option 3. After decision tree analysis, the expected value for undertaking policy option 2, was $ 24, policy option 3 was $ 856 per person traveller going through the airport (Table 2)
Table 2: Cost effective analysis for the three policy options for the airport screening 2020, Uganda
|
Outcome |
Option 1: No Screening No Testing
|
Option 2: Mandatory symptom screening for all, testing only the symptomatic | Option 3: Mandatory quarantine and Testing for all
|
| Cases that come in through airport | 3375 | 3375 | 3375 |
| Cases identified through the airport Program | – | 1,369 | 2,363 |
| Infected who end up in the community | – | 2,006 | 1,013 |
| Number of 1st generation cases | 12,150 | 5,722 | 3,195 |
| Expected value | – | $24 | $856 |
| Total Costs (US$) | – | $1,585,159 | $58,417,300 |
| IC | – | $1,585,159 | $58,417,300 |
| Cases averted | —- | 6,428 | 8,955 |
| ICER cost/case averted* | —- | $247 | $6,524 |
| Average cost | – | $277 | $18,281 |
*ICER: Incremental cost-effectiveness ratio, ICER = (Costs2-Costs1)/(Effectiveness2-Effectiveness1).
While comparing option 2 & 3 to option 1(no airport screening & testing), a total of 5,722 1st generation cases were identified with option 2, and 3,195 with option 3. Option 2 helped in averting a total of 6,428, cases and option 3 a total of 8,955 cases. The total cost for implementation of this option 2 was $1,585,159 for option 3 Intervention was $58,417,300 option 2 resulted in an Incremental cost-effectiveness of $247 per case averted, option 3 $6,524 per case averted. The average cost for each person undertaking option 2 and option 3 were $ 277 and $18,281 respectively (Table 2)
Policy Implications
First, the varying costs of testing, and the increase in the prevalence of COVID-19 could influence the cost-effectiveness ratio of with option 2 in this study. A study (quilty, B) showed that the effectiveness of entry screening is largely dependent on the effectiveness of the exit screening in place, could only detect 53 (95% CI: 35–72) instead of ninety infected travellers if no exit screening was in place, in addition, the wider use of Option2 in COVID-19 detection is an adopted national strategy, setting an appropriate and effective screening is a necessary first step.
Second, the wider use of Option 2 needs to be discussed in conjunction with ethical considerations, such as consequences of incorrect results (e.g., false positives), airport screening protocol violation, whether or not to use is it as single strategy will need to be supplemented by these measures like recordkeeping to aid contact tracing, risk communication for this policy option to be effective
Critique of policy options
The World Health Organization through the International Health Regulations (IHR) recommends strengthening capacities to detect, assess, notify, and report events (4). This requires a surveillance system in place to collect information which Uganda has already developed. Additionally, the regulation requires the country to have prompt mechanisms to quickly detect surges or disease outbreaks which is still weak due to the slow analysis of all surveillance data.
Policy recommendations
This study was a step towards determining associated costs of controlling disease outbreak, therefore, implementation of mandatory symptom screening for all, testing only the symptomatic incoming travellers’ strategy should be prioritized. Nevertheless, any future respiratory infectious disease outbreak caused by SARS-COV-2 or any unknown respiratory pathogen, will likely require similar control measures.
References
- Anderson, R. M., Heesterbeek, H., Klinkenberg, D., & Hollingsworth, T. D. “How will country-based mitigation measures influence the course of the COVID-19 epidemic?.” The Lancet, 395(10228), , 2020: 931-934.
- Chinazzi, M., Davis, J. T., Ajelli, M., Gioannini, C., Litvinova, M., Merler, S., … & Viboud, C. (2020). ” The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) .” outbreak Science, 2020: 395-400.
- Kringos, D., Carinci, F., Barbazza, E., Bos, V., Gilmore, K., Groene, O., … & De Lusignan, S. ” Managing COVID-19 within and across health systems: why we need performance intelligence to coordinate a global response.” Health Research Policy, 2020.
- Li, Q., Guan, X., Wu, P., Wang, X., Zhou, L., Tong, Y., … & Xing, X. “Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia.” New England Journal of Medicine., 2020.
- Migisha, R., Kwesiga, B., Mirembe, B. B., Amanya, G., Kabwama, S. N., Kadobera, D., … & Lubwama, B. “Early cases of SARS-CoV-2 infection in Uganda: epidemiology and lessons learned from risk-based testing approaches–March-April 2020.” Globalization, 2020
Estimating the cost of managing COVID-19 patients in Uganda from March to June, 2020
Authors: Aggrey Byaruhanga1*, Doreen N. Gonahasa1, Elizabeth Katana1, Phoebe Nabunya1, Alex Ndyabakira1, Richard Migisha1, Geoffrey Amanya1, Daniel Kadobera1, Alex Riolexus Ario1*, 1Uganda Public Health Fellowship Program, Kampala, Uganda
Summary
Management of COVID-19 patients is resource-intensive, and estimating its costs is complex. In a resource-limited setting such as Uganda, where the gross domestic product per capita (GDP) is $643, it is important to understand the cost of managing COVID-19 patients to facilitate public resource planning. We estimated these costs early during the pandemic in Uganda.
We reviewed health facility records at Mulago national referral hospital, 13 regional referral hospitals, and 3 district hospitals managing COVID-19 patients in Uganda to obtain direct and indirect costs related to management of COVID-19 patients from march to June, 2020. We used the data to calculate the average cost of managing a COVID-19 patient and identified cost drivers for managing COVID-19 patients.
Of 1,297 patients evaluated, 967 (99%) had mild (asymptomatic/minimally symptomatic) infection; 866 (77%) had no known co-morbidities. The mean time spent in the hospital per patient was 2.5 (range, 1-4) weeks. The total direct cost for the 1,297 patients was estimated at $2,361,044; indirect costs at $263,031. The average cost of managing a COVID-19 patient was $2,023. Healthcare supplies 8.6%), food and drinks for patients/health care workers (18.6%), and healthcare workers’ allowances (14.9%) were the main direct cost drivers for managing COVID-19 patients from March to June 2020.
The average cost of managing a COVID-19 patient in Uganda was high compared to the GDP despite nearly all patients having mild disease. More than half of the costs were for healthcare supplies. We recommend the Ministry of Health to do appropriate budgetary allocations to support COVID-19 response in Uganda.
Introduction
On January 30, 2020, WHO declared severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) a Public Health Emergency of International Importance(1). In response, World Health organization (WHO) appealed for US$675 million to sup- port member states over a 3-month period, as they began implementing priority public health measures (2).
As of August 5, 2020, more than 18 million cases of COVID-19, including more than 600 000 deaths, had been reported globally(3). By the end of July, COVID-19 pandemic had disrupted the global economy and placed an enormous burden on the healthcare system (4, 5). The WHO explicitly expanded the scope of the strategic preparedness and response plan to include a ninth pillar on the maintenance of essential health services in acknowledgment that the pandemic was already straining the health system(6).
The early experience in countries with large-scale community transmission such as China, Iran, Italy, and Spain showed that the management of COVID- 19 Cases required unprecedented mobilization of health care resources (7). Projections in the United States showed that the total cost of the epidemic could reach more than $650 billion if the majority of the population became infected with COVID-19 (8).
On 21 March, Uganda detected its first case of COVID-19 in a traveler from Dubai at Entebbe International Airport(9). By 29th July, the country had registered a total number of 1,147 of COVID-19 cases. The Ministry of Health (MoH) preparedness and response plan included key pillars, namely: co- ordination, logistics, surveillance and laboratory, case management and Infection prevention and control, risk communication and social mobilization, and mental health and psychosocial support, and management of COVID-19 patients in isolation centers (10). All the public health measures associated with these pillars require substantial financial and human resources to expedite the response.
There was inadequate information about the cost of managing COVID-19 patients in sub-Saharan Africa including Uganda. To better understand the cost implications of managing COVID-19 patients Uganda, we established the cost of managing COVID-19 patients in Uganda using cases admitted in COVID-19 treatments units (CTUs) across the country from March to June, 2020.
Methods Study design
We also reviewed patient files from the national referral hospital, the 13 regional referral hospitals, and the 3 district hospitals that were managing COVID-19 patients from March to June 2020 at the time. We defined a confirmed COVID-19 case as a patient with PCR positive results found in his or her file
Study variables and data collection
We reviewed files of all COVID-19 patients admit- ted in hospitals with COVID-19 in Uganda with complete information from march, 2020 and dis- charged by 31th June, 2020. We collected information on socio-demographic and clinical characteristics, length of hospital stay captured in weeks, laboratory tests performed, and the treatment given to patients.
The heads of COVID-19 treatment Units, records officers, accountants and hospital directors provided evidence of health care expenditure.
We collected information on direct and indirect costs (from patient files and health care supplies documents such as stock cards and delivery note books and receipts) incurred in the management of COVID-19 patients using a cost analysis spread sheet. Direct costs were defined as expenditure on managing a patient from the day of admission up to discharge. Direct medical costs included drugs and medical equipment and direct non-medical costs such as transportation costs and allowances. We collected data on transport costs (fuel, car service) for health workers travelling from workplace to their respective residents during total lock down, the cost of CTUs trainings and meetings, food and drinks for the hospital task forces during meetings and trainings, the diagnostic tests (laboratory and radiology), risk allowance for the front-line health workers working in CTUs, disinfection cost, food and drinks for both health workers and COVID-19 patients, utility bills (water and electricity) and the cost of medicines and supplies (Face masks, face shields, gum boots, theatre shoes, aprons among others).
The indirect costs were defined as the expenses incurred from the cessation or reduction of work productivity as a result of the morbidity from COVID-19. For indirect costs, we calculated the number of days spent by each patient in the hospital multiplied by the estimated amount of money patient she/he earns a day based on occupation. We excluded the depreciation of the buildings, vehicles or any assets involved in COVID-19 response.
We also identified cost drivers for managing COVID-19 patients and these were defined as those items that consumed most of the resources during the management of COVID-19 patients.
The main outcome of this cost evaluation was to estimate the total cost of managing COVID-19 patients in health care facilities in Uganda from ad-mitted from march to June, 2020.
Data analysis
Using MS Excel 2019, we calculated the total cost of managing COVID-19 patients (direct and indirect costs) in US Dollars. We calculated the direct and indirect costs in order to identify the cost drivers of managing COVID-19 patients in health care facilities in Uganda. We converted the Ugandan shillings to UD dollars at a rate of one US dollar to three thousand seven hundred and eleven Ugandan shillings (3711). Data on socio-demographic characteristics of COVID-19 patients was presented in percentages and table form.
Results
Characteristics of COVID-19 patients in Uganda from march to June, 2020
We reviewed records of 1,297 patients, almost half of patients 734(47.8%) spent 2 weeks in the hospital, majority 768 (67.5%) were asymptomatic at the time of admission, 266 (23.5 %) of patients had comorbidities, and 968 (99.0%) had mild COVID- 19 infection (table 1).
Table 1: Shows the clinical characteristics of COVI9-19 patients in Uganda from march to June, 2020
| Variable (N=1,297) | Frequency (n) | Percentage (%) |
| Hospital stay (N=1,297) | ||
| 1 Week | 102 | 3.3 |
| 2 Weeks | 734 | 47.8 |
| 3 Weeks | 345 | 33.7 |
| 4 Weeks | 116 | 15.2 |
| Symptoms at admission (N=1138) | ||
| Symptomatic | 370 | 32.5 |
| Asymptomatic | 768 | 67.5 |
| Comorbidity (N=1,132) | ||
| No | 866 | 76.5 |
| Yes | 266 | 23.5 |
| Severity of COVID-19 (N=977) | ||
| Mild | 967 | 99 |
| Moderate | 8 | 0.8 |
| Severe | 2 | 0.2 |
The cost of managing a COVID-19 patient in Uganda, March to June, 2020
The estimated the direct cost of managing COVID- 19 patients in Uganda from March to June, 2020 was at two million three hundred sixty-one thousand and forty-four Us Dollars (2,361,044), indirect cost at two hundred sixty-three thousand and thirty- seven US Dollars (263,037). While the total cost of managing COVID-19 patients was estimated at two million six hundred twenty-four thousand and eighty-one US dollars (2,624,084).
The established average cost of managing a COVID- 19 patient was two thousand and twenty-three US Dollars (2,023).
The cost drivers for managing COVID-19 patients in Uganda from March to June, 2020
Table 3: Total cost drivers for managing COVID -19 patients, March to June, 2020, Uganda
| Variable | Cost ($) | |
| HW allowances | 351,764 | 14.9 |
| Fuel | 137,116 | 5.8 |
| Food& Drinks | 438,885 | 18.6 |
| Utilities | 50,192 | 2.1 |
| Health supplies | 1,383,086 | 58.6 |
| Total | 2,361,044 | 100 |
More than half (58.6%) of the money was spent on health care supplies, 18.6% on food and drinks for health workers and patients, 14.9% on health worker allowances, 5.8% on fuel costs and the least 2.1% on utilities (water and electricity) (Table 2).
Discussion
Our cost evaluation was conducted in the first three months of the pandemic in Uganda where almost all COVID-19 patients were a symptomatic. Patients did not require confiscated medical diagnosis and treatment. All patients by the time of data collection were managed in designated hospitals (CTUs).
Our findings established that the average cost of managing a COVID-19 patient in Uganda from march to June, 2020 was $2,023. The overall cost (direct and indirect) was estimated at $2,624,081. This cost is likely to be low because, more than half (67.5%) of the COVID-19 patients were asymptomatic and 99.0% had mild COVID-19 infection. The cost is likely to increase due increased community transmission and many patients going into advanced stages of the disease, thus requiring intensive care treatments (11).
Health care supplies (including drugs, diagnostic tests and IPCs) were the main cost drivers accounting for 58.6% followed by food/drinks for both patients and health workers at 18.6% and health workers allowances at 14.9%. This agrees with the study conducted by Edejer T et al, 2020 in United states where health care supplies accounted for (54%), maintaining essential services at (21%), rap- id response and case investigation at (14%), and infection prevention and control at (9%) (11). Due to the dynamic transmission of SARS-CoV-2 through direct, indirect, or close contact with infected people through infected secretions such as saliva and respiratory secretions or their respiratory droplets, which are expelled when an infected person coughs, sneezes, talks or sings (2-10), health workers and patients use a lot of IPC measures such as masks, aprons, handwashing sanitizers, biohazard containers among others to protect themselves against the infection. It is necessary that during the subsequent budgetary allocations to management COVID-19 patients, the Ministry of Health should appropriate more funds to procurement of health care supplies.
Our findings also established that the overall cost of managing COVID-19 patients was generally high compared to other outbreaks in Uganda, such as Cholera outbreak in Hoima district(12) and measles outbreak in Buvuma district(13) with an overall cost of $71.769 and $16,259 respectively. The difference is due to the localized nature of the former outbreaks that were occurring in one district compared to SARS-CoV-2 which spread throughout the country. Our findings further established a high cost of managing a COVID-19 patient compared to other infectious diseases. For instance, a study conducted in West Africa found that the estimated cost of treating an Ebola case was ranging from $480 to $912(14). This high cost may be attributed to IPCs implemented in CTUs, such as use of masks by both patients and health workers, face shields and the cost of RT-PCR test for COVID-19 which is relatively high.
Conclusion
The average cost of managing a COVID-19 patient in Uganda was high compared to the GDP despite nearly all patients having mild disease. More than half of the costs were for healthcare supplies.
Study limitation
Due the retrospective nature of this study, most of the data from patient files, health facility records was missing which may have resulted in under estimation of the costs. Most of the patients were asymptomatic, they required only vitamin C and no supportive care. As a result, these should be understood as the cost of mild COVID-19 disease. Regardless, a cost of more than $2000 USD per patient with almost no clinical illness is relatively high. After easing lock down (August, 2020), we have evidenced increased community transmission with a high proportion of cases in Uganda having moderate to severe disease. The cost of managing such patients is likely to be too much higher because they will require confiscated medical diagnostics and treatment care. Therefore, this may affect generalizability of the findings in other re- source limited settings.
Recommendations
We recommend the Ministry of Health to do appropriate budgetary allocations to support COVID- 19 response in Uganda. We also recommend another cost evaluation to estimate the cost of managing COVID-19 cases in Uganda including those with severe disease
References
- WHO. WHO fact sheet: COVID-19 as a Public Health Emergency of International Concern (PHEIC) under the IHR. 2020. https:// extranet.who.int/sph/ sites/default/files/document -library/document/FS15A_IHR_OVID19_EN_MAY_2020.pdf (accessed June 17, 2020). 2020.
- WHO. US$675 million needed for new coronavirus preparedness and response global plan.
- https://www.who.int/news-room/detail/05-02-2020-us-675-million-needed-for-new-coronaviruspreparedness-and-response-global-plan (accessed June 17, 2020). 2020.
- WHO. WHO fact sheet: Corona Virus disease (COVID-19): Situation Report. 2020.
- Fernandes N. Economic effects of coronavirus outbreak (COVID-19) on the world economy. Available at SSRN 3557504. 2020.
- Chakraborty I, Maity P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Science of the Total Environment. 2020:138882.
- AlKhaldi M, Kaloti R, Shella D, Al Basuoni A, Meghari H. Health system’s response to the COVID -19 pandemic in conflict settings: Policy reflections from Palestine. Global public health. 2020;15 (8):1244-56.
- McKibbin WJ, Fernando R. The global macro- economic impacts of COVID-19: Seven scenarios 2020
- WHO. Strengthening the health system response to COVID-19-Recommendations for the WHO European Region Policy brief (1 April 2020). WHO Regional Office for Europe. 2020.
- MOH. COVID-19 daily situation a report,
- Ministry of Health, Kampala, Uganda. Kampala, Uganda: Ministry of Health; 2020.
- MOH. Analytical status of report 06: COVID- 19 in Uganda. 2020.
- Edejer TT-T, Hanssen O, Mirelman A, Ver- boom P, Lolong G, Watson OJ, et al. Projected health-care resource needs for an effective response to COVID-19 in 73 low income and middle-income countries: a modelling study. The Lancet Global Health. 2020.
- Okullo E, Zhu B-P. The cost of responding to a waterborne cholera outbreak in a village in Uganda compared to a simple hypothetical intervention. International Journal of Infectious Diseases. 2016;53:137.
- Nguna J, Bbaale JK, Birungi D, Kwesiga B, Kadobera D, Opar BT, et al. Costs of Responding to a Measles Outbreak: Buvuma Islands, Lake Victoria, Uganda, February-May 2017. 2019.
- Bartsch SM, Gorham K, Lee BY. The cost of an Ebola case. Pathogens and global health. 2015;109 (1):4-9.
DOWNLOAD FULL PDF ISSUE BELOW

Comments are closed.