Trends and distribution of Vibrio cholerae isolates at the National Microbiology Reference Laboratory, Ministry of Health, Uganda, 2014 –2023
Authors: Leah Naluwagga Baliruno*1, 2, Rita Namusoosa1, 2, Samuel Gidudu1, Paul Edward Okello1, Harriet Nakigozi2, Chris Okiria2, Isaac Ssewanyana2, Suzan Nabadda2, Grace Najjuka2, and Alex Riolexus Ario1 Institutional affiliations 1Uganda Public Health Fellowship Program, National Institute of Public Health, Kampala Uganda, Ministry of Health, Uganda, 2National Health Laboratories and Diagnostic Services (NHLDS), Ministry of Health, Uganda Correspondence*: Tel: +256702533694, Email: lnaluwagga@uniph.go.ug
Summary
Background: As per the World Health Organization, countries including Uganda are to end cholera by 2030 through prevention and treatment. This achievement can be hindered due to rapid changes in antimicrobial response patterns and serotype among other factors. We described confirmed cholera cases by person, place, time, serotype, antimicrobial resistance, and multi-antimicrobial-resistant phenotype patterns, Uganda, 2014–2023.
Methods: We conducted a descriptive study using the 2014 – 2023 data on confirmed cholera cases abstracted from the National Microbiology Reference Laboratory (NMRL) register. We described the cases by age group, sex, district, serotype, reporting period, antimicrobial resistance (resistant and intermediate)(rates), and multi-antimicrobial-resistant phenotype patterns. We described the confirmed cases and the antimicrobial resistance patterns over time. Mann-Kendall tests for trends were used to test the significance of AMR trends.
Results: We identified 489 confirmed cholera cases between January 2014 to December 2023 whose V. cholerae isolates were referred by 35 districts in Uganda. The majority of the identified confirmed cholera cases were male (239, 49%), aged 21-40 years (170, 38%), had V. cholerae 01 Ogawa (256, 52%) and were from Kampala District (138, 28%). We observed a gradual decline in confirmed cholera cases over time with peaks in 2015, 2018 and 2023. Vibro cholerae 01 ogawa was observed to dominate throughout the period. We observed consistent resistance by V. cholerae to 6 antimicrobials from 2014 to 2023. 194 (39.7%) isolates showed multiple antimicrobial-resistant with 90 (18.6%) resistant to more than one class of antimicrobials.
Conclusion: We observed males, persons aged 21-40 years, and Kampala District as being most affected with cholera in Uganda with peaks in 2015, 2018, and 2023 and Vibro cholerae 01 Ogawa as the predominate serotype. Consistent antimicrobial resistance was exhibited over time between 2014 and 2023. Intensifying cholera disease prevention by the Ministry of Health targeting males, persons aged 21-40 years, and Kampala District is critical. Routine antimicrobial surveillance to guide informed antimicrobial use and prevent the spread of AMR, especially during cholera outbreaks is important.
Background
Vibrio cholerae (V.cholerae), a gram-negative bacillus and the causative agent for cholera disease, an acute diarrheal illness transmitted mainly through the fecal-oral route. The serogroups of this bacteria include V. cholerae O1 and O139. V. cholerae O1 has serotypes Inaba and Ogawa (1). Cholera disease if not treated on time, leads to severe dehydration, shock (within 6 to 12 hours), and possibly death (2). Uganda has reported cholera outbreaks almost every year in the last two decades (3) with an average of 11,000 cholera confirmed cases annually, and 61 to 182 deaths each year (4). According to the United Nations Children’s Fund (UNICEF) children under 5 years are more affected by the disease (5).
During cholera outbreak investigations, stool samples are analyzed to confirm and monitor outbreak progression, particularly in endemic areas where sporadic cases and small outbreaks frequently occur (2). The collected data is then examined to identify the affected populations, outbreak locations, and timing. Additionally, the data is used to track changes in Vibrio cholerae serotypes and antimicrobial susceptibility patterns. Common antimicrobial agents employed in this context include tetracycline, doxycycline, trimethoprim-sulfamethoxazole (SXT), erythromycin, and chloramphenicol safeguarding the effectiveness of antimicrobial agents in the face of emerging resistance (6). We described confirmed cholera cases in Uganda by person, place, time, and serotype as well as characterize V.cholerae isolates by antimicrobial resistance trends and multi-antimicrobial-resistant phenotypes, Uganda, 2014-2023.
Methods
We conducted a descriptive study using the 2014 – 2023 data on confirmed cholera cases abstracted from the National Microbiology Reference Laboratory (NMRL) register. We described the cases by age group, sex, district, serotype, reporting period, antimicrobial resistance (rates), and multi-antimicrobial-resistant phenotype patterns. We further described trends of the confirmed cholera cases and the antimicrobial resistance patterns. The rates of antimicrobial resistance (AMR) were calculated as the proportion of resistant (resistant and intermediate) out of the total number of organisms tested for antimicrobial susceptibility per drug, per year. Antimicrobial resistance (AMR) rates for each antibiotic were calculated separately and AMR trends demonstrated using line graphs. Mann-Kendall tests for trends were used to test the significance of AMR trends.
This study utilized routinely collected aggregated confirmed cholera surveillance data associated with minimal risk to the individual to achieve the study objectives. We sought permission to access and use the data from the NMRL. The Centre for Global Health, US Centre for Disease Control and Prevention (US CDC) determined that this project was not human subject research and its primary intent was for public health practice or disease control, therefore it was classified as non-research. This study project was reviewed by the CDC and conducted consistent with applicable federal law and CDC policy.
Results
Description of cholera-confirmed cases by sex, age group, and serotype, Uganda, 2014-2023
We identified 489 confirmed cholera cases during January 2014 to December 2023 whose V. cholerae isolates were referred by 35 districts in Uganda.
The majority of the identified confirmed cholera cases were male (239, 49%), aged 21-40 years (170, 38%), and had V. cholerae 01 Ogawa (256, 52%) (Table 1). Most of these cases were from Kampala (138, 28%) (Figure 1).
Table 1: Description of cholera-confirmed cases by sex, age group, and serotype, Uganda, 2014-2023
| Table 1: Description of cholera-confirmed cases by sex, age group, and serotype, Uganda, 2014-2023 | ||
| Variable | Frequency (n=489) | Proportion (%) |
| Sex | ||
| Male | 239 | 48.9 |
| Female | 207 | 42.3 |
| Unknown | 43 | 8.8 |
| Age group (years) | ||
| ≤ 2 | 18 | 3.7 |
| 3-5 | 53 | 10.8 |
| 6-20 | 145 | 29.7 |
| 21-40 | 170 | 34.8 |
| 41-60 | 42 | 8.6 |
| ≥ 61 | 15 | 3.1 |
| Unknown | 46 | 9.4 |
| Serotype | ||
| O1 Inaba | 151 | 30.9 |
| O1 Ogawa | 256 | 52.3 |
| not serotyped | 82 | 16.8 |
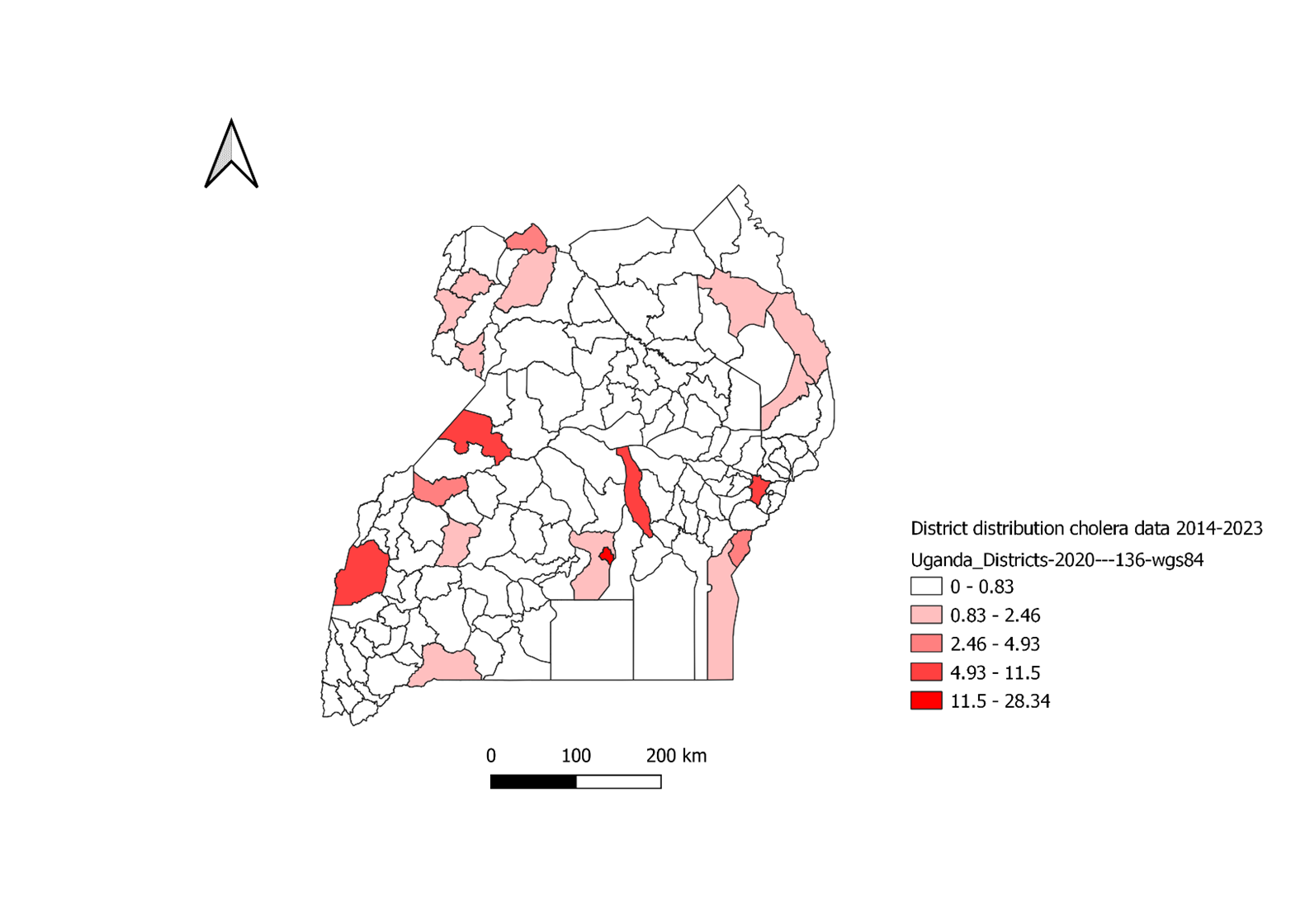
Number of cholera cases, Uganda, 2014-2023
The number of confirmed cholera cases gradually declined over time, with peaks in 2015, at 137 (28%), in 2018, at 109 (22%), and in 2023, at 23, 65 (13%) (Figure 2).
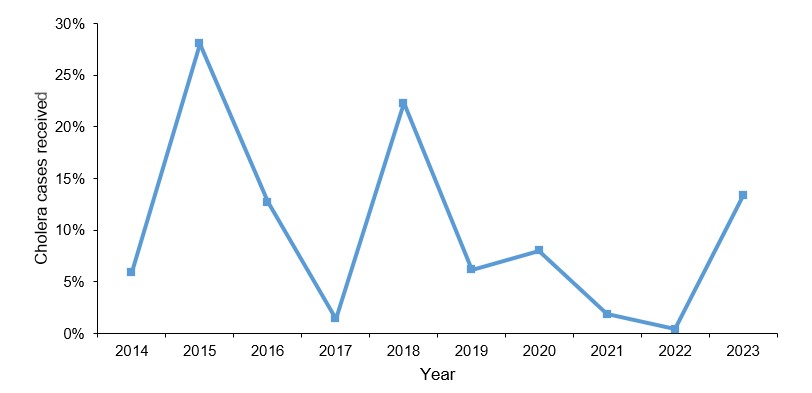
Trends of confirmed cholera confirmed cases by serotype, Uganda, 2014-2023
We observed a gradual decline in confirmed cholera cases over time with peaks in 2015, 2018, and 2023. Vibro cholerae 01 ogawa was observed to dominate throughout the period (Figure 3).
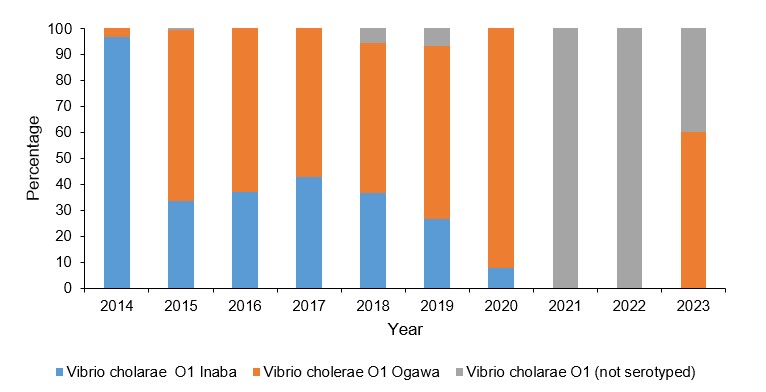
Trends of antimicrobial resistance of Vibro cholerae isolates, Uganda, 2014-2023
We observed consistent resistance by V. cholerae to 6 antimicrobials from 2014 to 2023. Increased resistance was mainly observed to nalidixic acid (100%, p=1.000), ciprofloxacin (1.3%– 85%, p=0.2207) and trimethoprim-sulfamethoxazole (SXT) (31.2 – 52.6%, p=0.0864). There was decreased V. cholerae resistance for tetracycline, (7.5 – 1.8% p=0.0864) and chloramphenicol, (36.3– 0% p=0.0864) (Figure 4).
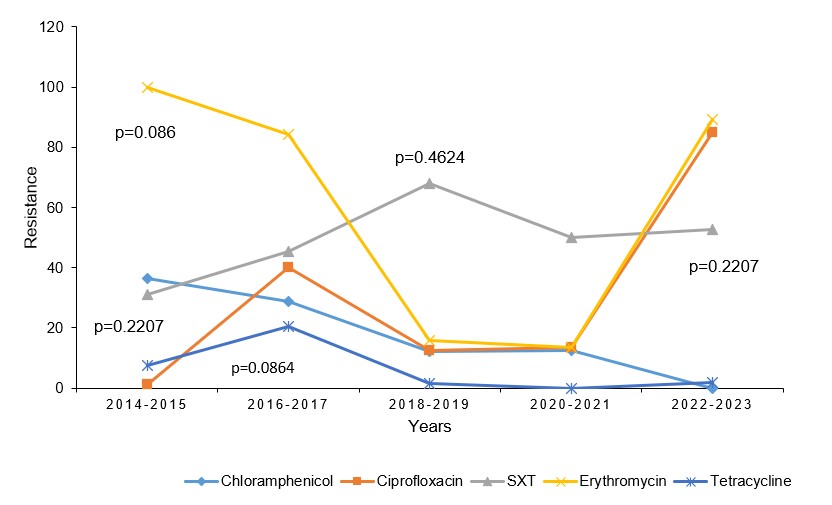
Distribution of V. cholerae by multiple antimicrobial-resistant phenotypes, Uganda, 2014-2023
Out of 489 V. cholerae isolates, 194 (39.7%) were multiple antimicrobial-resistant with 90 (18.6%) resistant to more than one class of antimicrobials. The most common resistotype was exhibiting simultaneous resistance to erythromycin, nalidixic acid, and SXT, 49 (10%) (Table 2).
Table 2: Multiple antimicrobial-resistant phenotypes of V. cholerae by class, Uganda, 2014-2023
| Property: MDR Phenotype | Frequency (n=489) | Percentage (%) | |
| Resistance to 5 classes of antimicrobials: | |||
| Chloramphenicol, erythromycin, nalidixic acid, tetracycline, SXT | 2 | 0.4 | |
| Resistance to 4 classes of antimicrobials: | |||
| Chloramphenicol, erythromycin, tetracycline, SXT | 11 | 2.2 | |
| Chloramphenicol, erythromycin, nalidixic acid, tetracycline | 2 | 0.4 | |
| Ciprofloxacin, erythromycin, nalidixic acid, SXT | 2 | 0.4 | |
| Chloramphenicol, ciprofloxacin/ nalidixic acid, erythromycin, SXT | 1 | 0.2 | |
| Resistance to 3 classes of antimicrobials: | |||
| Erythromycin, nalidixic acid, SXT | 49 | 10 | |
| Chloramphenicol, nalidixic acid, SXT | 25 | 5.1 | |
| Chloramphenicol, erythromycin, SXT | 2 | 0.4 | |
| Erythromycin, tetracycline, SXT | 2 | 0.4 | |
| Nalidixic acid, tetracycline, SXT | 1 | 0.2 | |
| Ciprofloxacin, erythromycin, SXT | 1 | 0.2 | |
| Erythromycin, nalidixic acid, tetracycline | 1 | 0.2 | |
Discussion
Our study reported on the trends of V. cholerae serotypes and antimicrobial susceptibility in Uganda from 2014 to 2023. We identified males, persons aged 21-40 years, and Kampala District as being the most affected with peaks in 2015, 2018, and 2023 and Vibro cholerae 01 Ogawa as the predominate serotype. Consistent antimicrobial resistance was exhibited over time between 2014 and 2023.
- cholerae 01 Ogawa was pre-dominant over the years likely because of ongoing public health challenges associated with inadequate water and or sanitation infrastructure (18). This finding is similar to other studies that have also identified the v.cholerae 01 Ogawa as a pre-dominant serotype in Zambia which accounted for 70% of the 2009 and 2016 outbreaks (19).
The observed increase in antimicrobial resistance to V.cholerae could be associated with widespread and inappropriate use of antibiotics (20); the acquisition of resistant genes through horizontal gene transfer, a process that allows the bacterial to adapt to the presence of antibiotics (29) rapidly; exchange of plasmids that carry resistance genes between different strains (17); environmental factors such as contaminated waters sources that act as reservoirs for resistant bacteria (21) and lack of adequate surveillance of antibiotic resistance in the country (22).
Study limitations
Our data source was characterized with missing and incomplete information due to frequent stockouts of antimicrobial disks and antisera. Thus leading to either under or over estimation of the study outcomes and limiting the generalizability of the findings.
Conclusion
Our study reveals a disproportionate burden of cholera among males, young adults, and Kampala residents, with Vibro cholerae 01 Ogawa as the predominant serotype, recurring outbreaks and persistent antimicrobial resistance. These results highlight the need for targeted interventions and enhanced surveillance to address the ongoing cholera epidemic in Uganda. The consistent antimicrobial resistance observed over time underscores the importance of antibiotic stewardship and alternative treatment strategies.
The Ministry of Health should prioritize targeted cholera prevention measures for high-risk groups, including males, persons aged 21-40, and Kampala District through improving sanitation and hygiene measures. Continuous antimicrobial surveillance is also crucial to inform evidence-based treatment decisions and mitigate antimicrobial resistance.
Conflict of interest
None
Authors Contribution
LNB, RN, PO, HN, and SG conceived and designed the analysis. LNB and RN collected the data. LNB, RN, PO, and HN contributed to the data analysis. LNB and RN performed data analysis. LNB, RN, PO, HN, and SG wrote the bulletin. IS, SN, GN, and AA reviewed the bulletin to ensure scientific integrity.
Acknowledgements
We acknowledge the Uganda Public Health Fellowship Program and the National Microbiology and Reference Laboratory (NMRL) for the technical support and oversight of this project. We acknowledge the US Centers for Disease Control and Prevention Uganda thorugh Makerere University School of Public Health, and Baylor Uganda for implementation support.
Copyright and licensing
All materials in the Uganda Public Health Bulletin are in the public domain and may be used and reprinted without permission. Citation as to the source is appreciated. Any article can reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- Alsamarai MA, Cheesbrough M. BOOK REVIEW Title: District Laboratory Practice in Tropical Countries Part 1 Second Edition Author. IJMS January. 2018;1(1):65–8.
- Allen G, Aj N, Chowdhury F, Ross AG, Islam T, Mcmillan NAJ. Diagnosis, Management, and Future Control of Cholera. Clinical Microbiology Reviewa. 2022;
- Bwire G, Munier A, Ouedraogo I, Heyerdahl L, Komakech H, Kagirita A, et al. Epidemiology of cholera outbreaks and socio-economic characteristics of the communities in the fishing villages of Uganda: 2011-2015. PLoS Neglected Tropical Diseases. 2017;11(3):2011–5.
- Bwire G, Malimbo M, Maskery B, Kim YE, Mogasale V, Levin A. The burden of cholera in Uganda. PLoS neglected tropical diseases. 2013;7(12):e2545.
- UNICEF. Cholera is endangering children globally [Internet]. Available from: https://www.unicef.org/stories/cholera-is-endangering-children-globally
- Khan AI, Chowdhury F, Harris JB, Larocque RC, Faruque ASG, Ryan ET, et al. Comparison of clinical features and immunological parameters of patients with dehydrating diarrhoea infected with Inaba or Ogawa serotypes of Vibrio cholerae O1. Scandinavian Journal of Infectious Diseases. 2010;42(1):48–56.
- Humphreys H, Fitzpatick F, Harvey BJ. Gender differences in rates of carriage and bloodstream infection caused by methicillin-resistant Staphylococcus aureus: are they real, do they matter and why? Clinical Infectious Diseases. 2015;61(11):1708–14.
- المنصوري نخك. Study of Vibrio Cholerae with its Virulence Factors Isolated from Diarrheal Patients in Babylon Province. 2017;
- Ngere P, Langat D, Ngere I, Dawa J, Okunga E, Nasimiyu C, et al. A protracted cholera outbreak in Nairobi City County accentuated by mass gathering events, Kenya, 2017. PloS one. 2024;19(8):e0297324.
- Endris AA, Addissie A, Ahmed M, Abagero A, Techane B, Tadesse M. Epidemiology of cholera outbreak and summary of the preparedness and response activities in Addis Ababa, Ethiopia, 2016. Journal of Environmental and Public Health. 2022;2022(1):4671719.
- Maponga BA, Chirundu D, Gombe NT. Cholera: a comparison of the 2008-9 and 2010 outbreaks in Kadoma City, Zimbabwe. Pan Afr Med J 20: 221. 2015.
- Chaudhary N, Biswas T, Ghosh P, Chattopadhyay S, Mondal R. Emergence of Multidrug Resistant Vibrio cholerae O139 in Acute Diarrhoea Patients Attending a Tertiary Care Hospital, West Bengal, India. JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH. 2023;17(1):DC14–8.
- Ismail S. ’Informal Settlements’a Great Threat to Uganda’s Realization of Sustainable Development: A Case Study of Slums in Kampala Capital City/Uganda. Ismail, S(2020)’Informal Settlements’a Great Threat to Uganda’s Realization of Sustainable Development: A Case Study of Slums in Kampala Capital City/Uganda International Journal of Humanities, Arts and Social Sciences. 2020;6(5):195–201.
- Eurien D, Mirembe BB, Musewa A, Kisaakye E, Kwesiga B, Ogole F, et al. Cholera outbreak caused by drinking unprotected well water contaminated with faeces from an open storm water drainage: Kampala City, Uganda, January 2019. BMC Infectious Diseases. 2021;21:1–9.
- Vermeiren K, Van Rompaey A, Loopmans M, Serwajja E, Mukwaya P. Urban growth of Kampala, Uganda: Pattern analysis and scenario development. Landscape and urban planning. 2012;106(2):199–206.
- Bwire G, Waniaye JB, Otim JS, Matseketse D, Kagirita A, Orach CG. Cholera risk in cities in Uganda: understanding cases and contacts centered strategy (3CS) for rapid cholera outbreak control. Pan African Medical Journal. 2021;39(1).
- Rijal N, Acharya J, Adhikari S, Upadhaya BP, Shakya G, Kansakar P. Changing epidemiology and antimicrobial resistance in Vibrio cholerae : AMR surveillance findings ( 2006 – 2016 ) from Nepal. 2019;1–8.
- Iramiot JS, Rwego IB, Kansiime C, Asiimwe BB. Epidemiology and antibiotic susceptibility of Vibrio cholerae associated with the 2017 outbreak in Kasese district, Uganda. BMC public health. 2019;19:1–9.
- Mwape K, Kwenda G, Kalonda A, Mwaba J, Lukwesa-Musyani C, Ngulube J. Characterisation of Vibrio cholerae isolates from the 2009, 2010 and 2016 cholera outbreaks in Lusaka province, Zambia. Pan Afr Med J. 2020; 35: 1–10.
- Ilić K, Jakovljević E, Škodrić-Trifunović V. Social-economic factors and irrational antibiotic use as reasons for antibiotic resistance of bacteria causing common childhood infections in primary healthcare. European journal of pediatrics. 2012;171:767–77.
- Uppal B, Mehra B, Panda PS, Kumar SK. Changing epidemiology and antimicrobial resistance pattern of Vibrio cholerae isolates at a tertiary care health laboratory in North India (2011–2015). water supply. 2017;4:6.
- Mohammed Y, Aboderin AO, Okeke IN, Olayinka AT. Antimicrobial resistance of Vibrio cholerae from sub-Saharan Africa: A systematic review. African Journal of Laboratory Medicine. 2018;7(2):1–7.


Comments are closed.