Trends and distribution of Malaria in Pregnancy, Uganda: analysis of surveillance data, 2015–2023
Authors: Charity Mutesi1*, Richard Migisha1, Lilian Bulage1, Jane Nabakooza2, Kasule Mathias2, Gerald Rukundo 2,Alex Riolexus Ario1; Institutional affiliations: 1Uganda Public Health Fellowship Program, Uganda National Institute of Public Health, Kampala, Uganda; 2National Malaria Control Division, Ministry of Health, Kampala, Uganda; *Correspondence: Tel: +256788626689, Email: charitymutesi@uniph.go.ug
Summary
Background: Malaria in Pregnancy (MiP) is associated with an increased risk of feto-maternal adverse outcomes. Pregnant women with malaria are at a higher risk for severe anemia and maternal death. We evaluated the trends and distribution of MiP from 2015–2023 to inform interventions to reduce MiP in Uganda.
Methods: We analyzed MiP surveillance data generated from the entire country in the District Health Information System (DHIS2), 2015–2023. MiP was defined as laboratory-confirmed malaria in a pregnant mother. MIP was computed as a proportion of pregnant women with malaria who attended ANC1. Mann-Kendall test was used to evaluate the significance of linear trends.
Results: A total of 2,808,426 MiP cases were reported during 2015–2023. The overall MiP incidence increased from 15% in 2015 to 21% in 2023(p=0.02). The incidence of MiP among women aged 10-19 years increased from 29% to 34% (p=0.013) while for those ≥20 years increased from 15% to 18% (p=0.017). Among the 15 regions of Uganda, Busoga (p=0.001), Teso (p=0.029), and West Nile (p=0.003) had increasing trends in MiP while the Karamoja region had a decreasing trend (p=0.0059). Incidence of MiP increased at Health Center III (p=0.029) whereas Regional Referral Hospitals reported a decreasing trend (p=0.036). An increasing trend of MiP was noted at public health facilities (p=0.029).
Conclusion: The incidence of MiP in Uganda increased during 2015–2023 suggesting higher risks of malaria-related complications during pregnancy. The burden of MiP was much higher among younger mothers at Health Centre III and public health facilities. Further investigation into the reasons for an increasing incidence of MiP in Busoga, Teso, and West Nile could provide insights for programming to reduce the burden.
Background
Malaria remains a disease of public health importance and has been on the global agenda for decades. Malaria infection carries serious risks for pregnant women, their fetuses, and their newborn babies. Pregnant women are at higher risk of malaria-related complications and death than age-matched women who are not pregnant (1,2). Infection of malaria during pregnancy is associated with an increased risk of fetus low birthweight, stillbirth, pre-term deliveries, miscarriages, maternal anemia, and sometimes maternal death; women in their first and second pregnancies are particularly vulnerable and decrement in intrauterine fetal growth (3,4).
The World Health Organization (WHO) estimated a global prevalence of 241 million cases in 2020 where sub-Saharan Africa took the top spot with more than 95 % of the worldwide burden (5). Similarly, there were an estimated 445,000 malaria deaths worldwide with 91% of the deaths occurring in the Africa region (6). The World Malaria Report, released in December 2021, reflects the global malaria community’s unique challenges. The report showed the devastating toll of malaria, with an estimated 627,000 people losing their lives to the disease in 2020 (7). In 2019, an estimated 11.6 million pregnancies were exposed to malaria in sub-Saharan Africa (SSA) which led to an estimated 822, 000 malaria in pregnancy (MiP)-related LBW babies and more than 40% of maternal anemia cases due to malaria occurring in these countries (7).
Malaria prevalence among pregnant women ranges from 13.1 to 50.0% but can be as high as 80% in highly endemic regions (8,9). The prevalence of MiP from population-based studies in Uganda ranges from 8.9% in the country’s low transmission areas to over 50% in high transmission settings (8–10). In Uganda, the overall burden of malaria is high and its adverse outcomes to the infected mother and the unborn child are widespread. In 2019, 152 of the total 3,528 maternal and newborn deaths in Uganda were due to MiP (9).
Risk factors associated with MiP include infection with human immunodeficiency virus (HIV), poor housing, low level of maternal education, and non-adherence to malaria preventive measures like insecticide-treated bed nets and IPTp (9,11–14).
Uganda has embarked on several interventions aimed at the prevention and reduction of morbidity and mortality due to MiP such as: having at least 60% of all pregnant women receive ≥2 doses of sulfadoxine-pyrimethamine (SP) as Intermittent Preventive Treatment during pregnancy (IPTp) in their second and third trimesters; at least 80% of pregnant women accessing quality case management; and at least 60% using Insecticide-treated bed nets (ITNs) (15). Use of ITNs has been reported to underlie a 23% reduction in placental parasitemia while ≥ 3 doses of IPTp-SP has been associated with up to 56% reduction in the risk of peripheral parasitemia and a significant decrease in sub-microscopic infections (16,17).
Despite the interventions, there has been a considerable increase in malaria transmission in Uganda, especially during the study period but it remains unclear how this has impacted the burden of MiP (18,19). Data analysis is important to help evaluate, directly or indirectly, the effectiveness of many years of targeted preventive interventions including IPTp-SP and ITNs. Identifying where greater burdens persist for intensifying control interventionsis useful. We examined trends and spatial distribution of malaria in pregnant women attending antenatal care in Uganda, 2015–2023.
Methods
Study setting
Malaria prevention and treatment services are integrated into routine antenatal care visits. MiP services are offered at various levels of healthcare facilities in Uganda, including primary health centers which are the first point of contact for most mothers and provide basic MiP services, district hospitals which offer more specialized care and serve as referral centers for primary health centers, and regional referral hospitals that provide advanced care and handle complex cases. The healthcare facilities are either government-owned where free MiP services are offered or privately owned where mothers pay a fee to access services. The country is divided into 15 non-administrative sub-regions comprising nine to thirteen districts considered the malaria endemicity zones under the Uganda Demographic and Health Survey program by 2018. The risk of malaria transmission in Uganda varies geographically, from less than 1% malaria prevalence in Southwest Uganda) to greater than 20% in Busoga, Northwest, and 34% in Northeast Uganda (20).
Study design and data source
We conducted a descriptive study using routinely collected MiP surveillance data in DHIS2. DHIS2 collects data on diseases of public health importance such as malaria based on the Integrated Disease Surveillance and Response guidelines (MoH 2021). It includes data on laboratory-confirmed cases due to MiP. In this study, we utilized aggregate data on MiP from monthly outpatient reports (Health Management Information System forms [HMIS] 105) 2015–2023.
Study variables, data distribution, and analysis
We extracted data on annual first antenatal visits and annual antenatal clinic malaria in pregnancy cases. In addition, we extracted data on HMIS 105 reporting rates. Malaria in Pregnancy (MIP) was computed as the proportion of pregnant women attending ANC1 who tested positive for malaria. We analyzed MiP incidence by examining the trends across several stratifying factors: geographic region, health facility level, facility ownership, and age of the pregnant women. For the age group variable, we considered data for 2020–2023 given that from 2015-2019, this variable was aggregated as <5 and >5 years which could not give meaningful analysis results. The age group variable was analyzed quarterly for the period 2020-2023 with the following age categories: 10-19 years and 20+ years. We used line graphs to demonstrate the trends and the Mann-Kendall test to determine the statistical significance of the trends. Data was analyzed using STATA version 17. Choropleth maps for MiP for the period from 2015 to 2023 were generated using Quantum Geographic Information System (QGIS) to indicate the distribution of MiP in Uganda.
Ethical Considerations
Our study utilized routinely aggregated surveillance data with no personal identifiers in health facility outpatient and in-patient monthly reports, obtained from the DHIS-2. The Uganda Public Health Fellowship Program is part of the National Rapid Response Team and has been granted permission to access and analyze surveillance data in the DHIS-2 and other data such as survey and field investigation data to inform decision-making in the control and prevention of outbreaks and public health programming. Additionally, the Ministry of Health (MoH) has also granted the Program permission to disseminate the information through scientific publications. We stored the abstracted dataset in a password-protected computer and only shared it with the investigation team. In addition, the Office of the Associate Director for Science, U.S. 144 Centers for Disease Control and Prevention, determined that this study was not a human subjects research with the primary intent of improving the use of surveillance data to guide public health planning and practice. This activity was reviewed by the CDC and was conducted consistent with applicable federal law and CDC policy. § 149 §See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Results
Trends in Malaria in Pregnancy among women attending antenatal care, Uganda, 2015–2023
Overall, 2,808,426 MiP cases were reported for the nine years reviewed. The incidence of MiP as a proportion of pregnant women attending antenatal care increased from 154/1,000 in 2015 to 205/1,000 in 2023(p=0.02). The highest incidence was in 2022 (218/1,000). The reporting rates were between 80% and 98% throughout the study period (Figure 1).
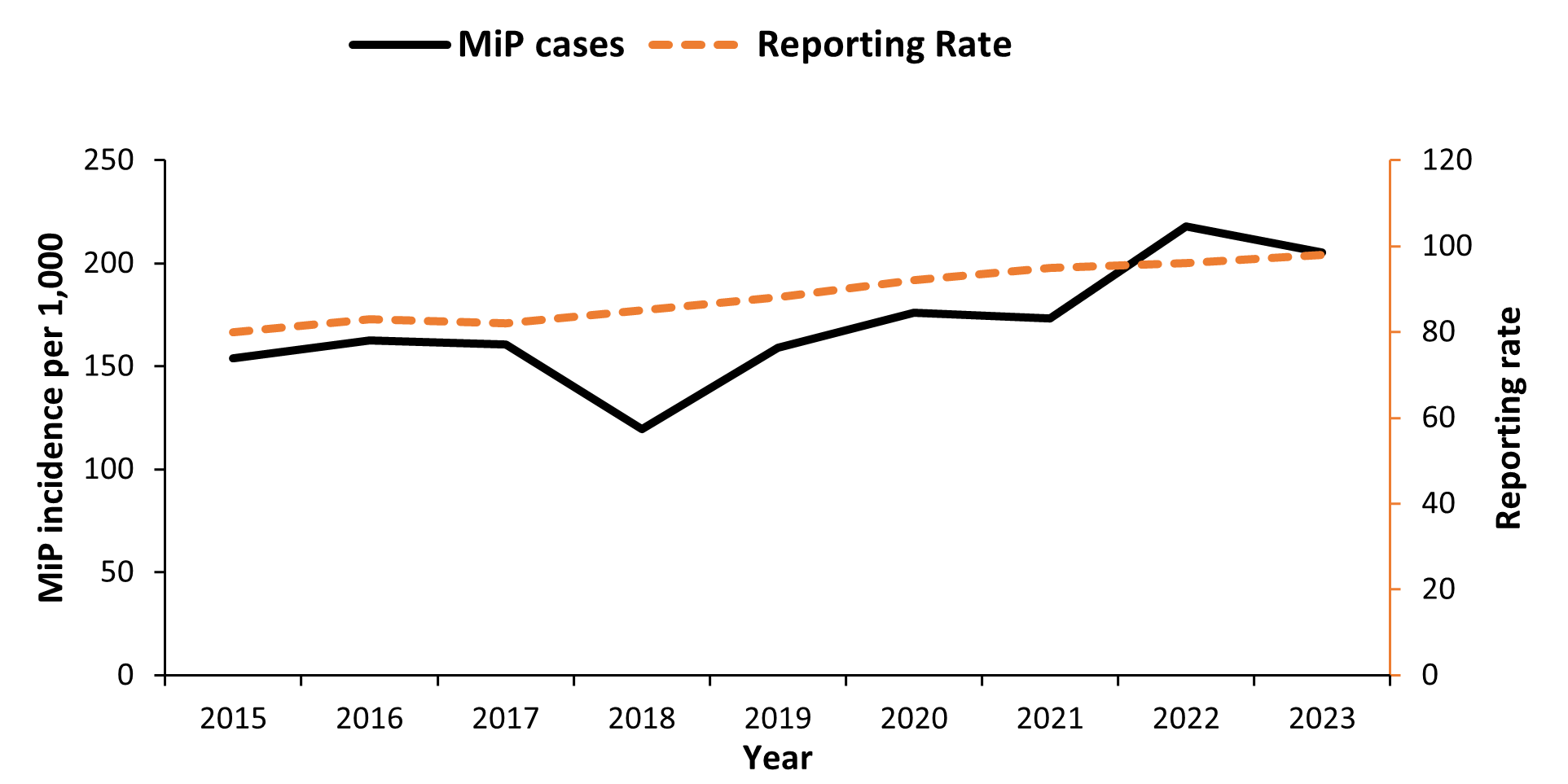
Trends of Malaria in Pregnancy among pregnant women attending antenatal care by health region, Uganda, 2015–2023
At the regional level, Busoga, West Nile, and Teso had a significantly increasing trend of MiP. However, the Karamoja region had a decreasing trend (Table 1).
Table 1: Trends of Malaria in Pregnancy among pregnant women attending antenatal care by Health Region, Uganda, 2015–2023
| Region | Kendall’s score | p-value |
| Busoga | 32 | 0.0012 |
| West Nile | 25 | 0.012 |
| Bunyoro | 23 | 0.76 |
| Teso | 22 | 0.029 |
| Lango | 18 | 0.076 |
| North Central | 14 | 0.18 |
| Bukedi | 8 | 0.47 |
| Acholi | 4 | 0.76 |
| Bugisu | 2 | 0.92 |
| Kampala | -3 | 0.83 |
| Tooro | -6 | 0.60 |
| Ankole | -8 | 0.47 |
| Kigezi | -10 | 0.34 |
| South Central | -10 | 0.34 |
| Karamoja | -19 | 0.0059 |
Trends of Malaria in Pregnancy among pregnant women attending antenatal care by age group, Uganda, 2015-2023
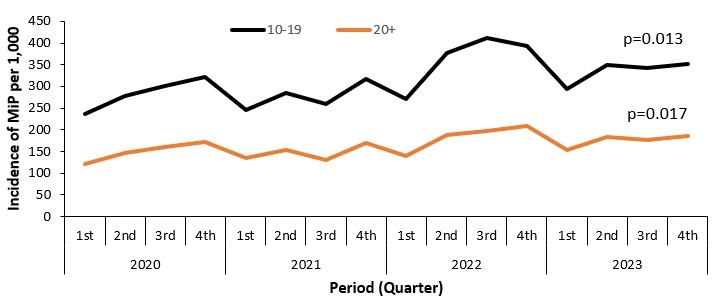
There was an increasing trend in the incidence of MiP in all age groups of pregnant women attending antenatal carei.e. 10-19 years (Kendall’s score=56, p=0.013) and those that were ≥20 years (Kendall’s score=54, p=0.017) (Figure 2).
Trends of Malaria in Pregnancy among pregnant women attending antenatal ca by health facility level, Uganda, 2015–2023
The proportion of MiP increased significantly in Health Centre III (HCIII) (p=0.02, Mk=22), while in the Regional Referral Hospitals (RRH), the proportion of MiP reduced significantly (p=0.03, Mk=-21). Among the other health facility levels (General hospitals, Health Centre IV, Health Centre II, and clinics), MiP proportions did not significantly change over the study period (Figure 3).
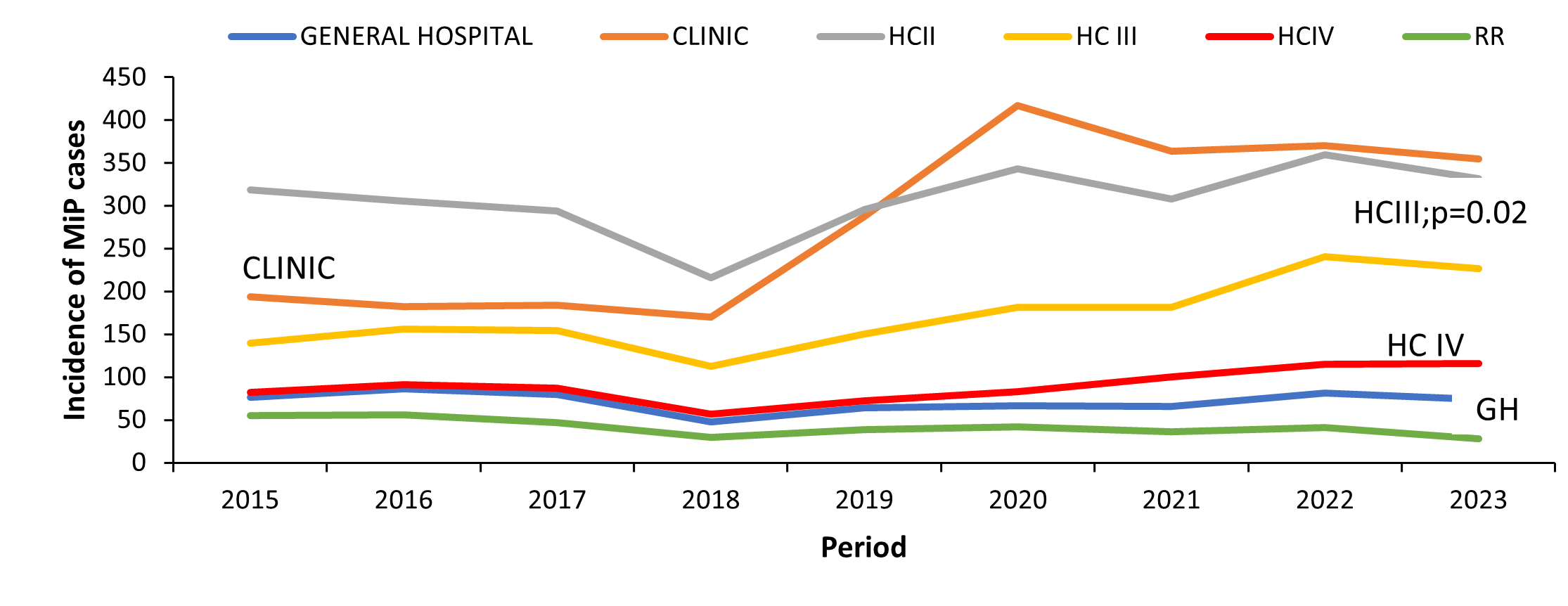
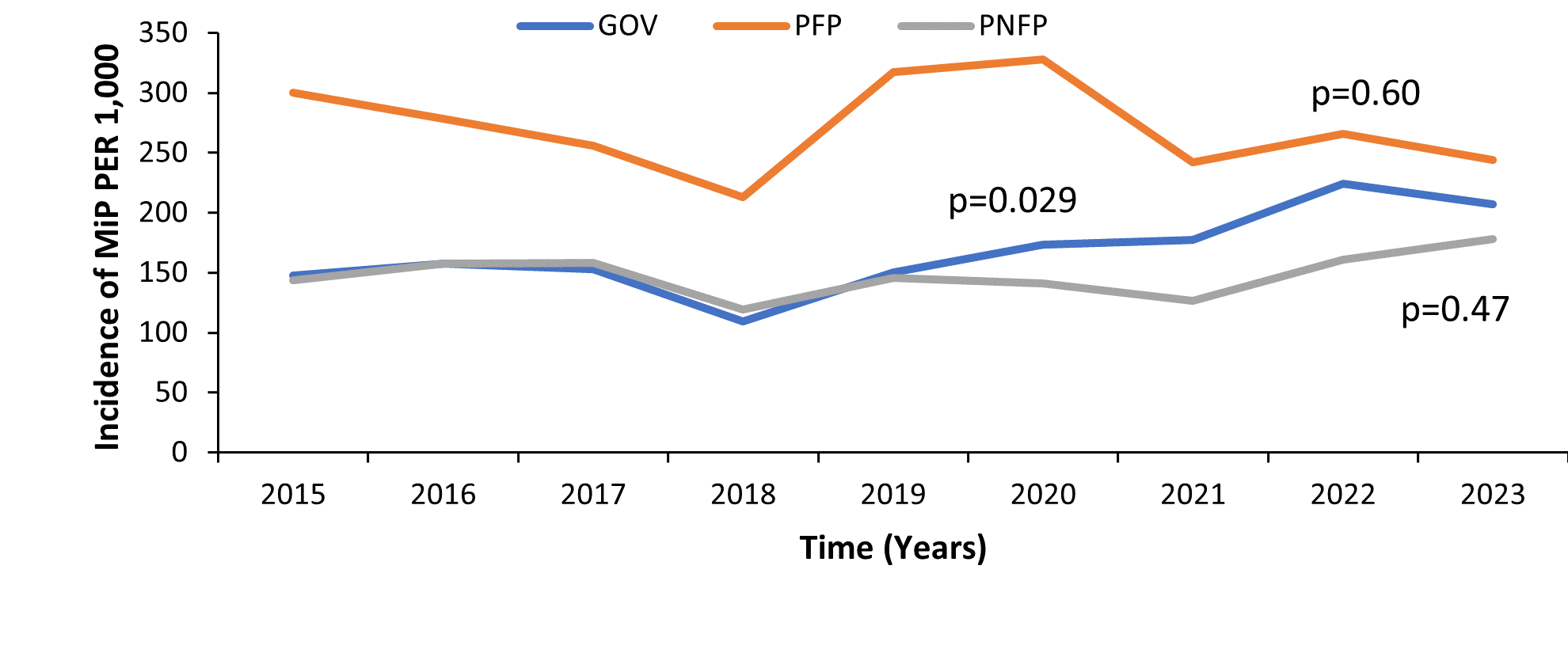
Trends of Malaria in Pregnancy among women attending antenatal care by Health Facility Ownership, Uganda, 2015–2023
The proportion of MiP significantly increased in government health facilities. In these facilities, the incidence of MiP increased from 147 cases per 1,000 pregnant women in 2015 to 207 cases per 1,000 pregnant women in 2023 (Figure 4).
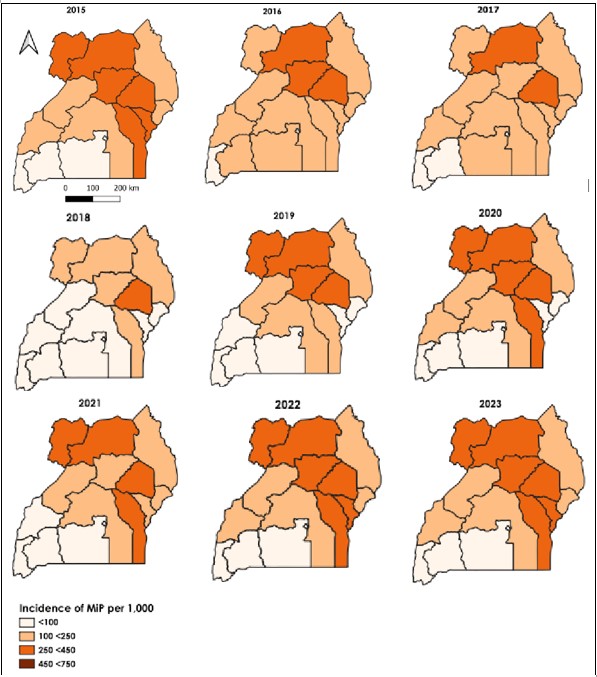
Spatial distribution of Malaria in Pregnancy among pregnant women attending antenatal care by health region, 2015–2023
There was an overall increase in MiP as a proportion of all pregnant women attending antenatal care. Increasing trends in MiP were observed in Busoga, West Nile, and Teso regions while decreasing trends were evident for the Karamoja region (Figure 5).
Discussion
We highlight an overall increase in the proportion of MiP among pregnant women attending antenatal care over the nine years nationally and for some of the regions. There was a significant decline in the trend of MiP in the Karamoja region. An increasing trend was also observed in all age categories of pregnant mothers with women within the age category of 10-19 years having a higher incidence of MiP than those who were ≥20 years. Looking at health facility type, only Health Centre III’s had an increasing trend while Regional Referral Hospitals depicted a decreasing trend. Public health facilities reported a significant increase in MiP during the study period.
The apparent increase in MiP incidence mirrors reported increases in the overall malaria burden in Uganda during the study period, a trend observed in other malaria high-burden countries (19). The notable increase from 2021 to 2022 in MiP cases reflected the rebound malaria epidemic, probably explained by the waning effects of the ITNs from the 2020 mass campaign. In addition, ITN distribution during the ANC1 visit was below 50% much less than the Uganda Malaria Reduction and Elimination Strategic Plan (UMRESP) (21).
The proportion of young pregnant women <20 years with MiP who could be primigravidae was significantly higher than those that were ≥20 years. Current evidence shows that the risk of malaria is higher in the primigravidae in younger pregnant women, and infections tend to peak early and decline towards term, reflecting the gradual acquisition of malaria immunity (11,12). In a Malawian study, adolescent pregnant women (age<20) were more likely to suffer from malaria than older women, and the risk was highest in the early trimester (23). Additionally, younger mothers have naïve pregnancy experience with limited knowledge and access to antenatal care where they would benefit from preventive interventions.
Health Center III’s and other lower-level facilities were found to have a significant increase in the trend of MiP incidence among other health facility levels. These findings are not surprising given that lower-level facilities are the first point of care for the mothers where they seek services for uncomplicated malaria (24). Equipping low-level health facilities with adequate malaria commodities for the management of MiP could help improve access to them.
Our findings revealed an increase in the trend of MiP in government health facilities unlike private-for-profit and private-not-for-profit health facilities. A study conducted on patient perspectives on interpersonal aspects of healthcare and patient-centeredness at primary health facilities revealed that patients using public facilities utilized them because of their proximity and the absence of fees charged at the time and point of use (24). A higher burden of disease has been noted among the poor with less access to healthcare services, this could explain the higher increase in the proportion of MiP in public health facilities that offer free medical services(24). Equipping public health facilities with adequate quantities of MiP management supplies is critical in ensuring the proper management of patients and reduced severity of MiP.
Study limitations
Our study has some limitations that should be considered when interpreting the results. We used secondary data from the District Health Information System 2 (DHIS2), which has challenges of completeness and accuracy, likely leading to either over or underestimation of MiP among pregnant women.
Conclusion
Uganda registered a significant increase in malaria in pregnancy incidence from 2015–2023 suggesting higher risks of malaria-related complications during pregnancy. The burden of MiP was much higher among younger mothers suggesting targeted interventions to this more vulnerable age group. MiP also increased at Health Centre III and public health facilities. Further investigation into the reasons for the increasing incidence of malaria in pregnancy in regions of Busoga, Teso, and West Nile could provide insights for programming to reduce the burden.
Conflict of interest
The authors declare that they have no conflict of interest
Author’s contribution
CM conceptualized the idea, analyzed and interpreted the data, and drafted the manuscript; RM, BK, LB, DK, GR, JN, MK, and ARA reviewed the bulletin for intellectual content.
Acknowledgments
We thank the staff of the Public Health Fellowship Program for the technical support and guidance offered during this study. We thank the Ministry of Health Malaria Control Division for raising the questions that initiated this analysis and overall guidance to the team.
Copyright and licensing
All material in the Uganda Public Health Bulletin is in the public domain and may be used and reprinted without permission. However, citation as to the source is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- Fried M, Duffy PE. Malaria during pregnancy. Cold Spring Harb Perspect Med. 2017;7(6):a025551.
- Desai M, Hill J, Fernandes S, Walker P, Pell C, Gutman J, et al. Prevention of malaria in pregnancy. Lancet Infect Dis. 2018;18(4):e119–32.
- Saito M, Briand V, Min AM, McGready R. Deleterious effects of malaria in pregnancy on the developing fetus: a review on prevention and treatment with antimalarial drugs. Lancet Child Adolesc Health. 2020;4(10):761–74.
- Takem EN, D’Alessandro U. Malaria in pregnancy. Mediterr J Hematol Infect Dis [Internet]. 2013 [cited 2024 Aug 2];5(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3552837/
- Organization WH. World malaria report 2023 [Internet]. World Health Organization; 2023 [cited 2024 Aug 2]. Available from: https://books.google.com/books?hl=en&lr=&id=u6UOEQAAQBAJ&oi=fnd&pg=PR6&dq=World+malaria+report,+2013+world+health+organisation&ots=cvnSk2Rmkl&sig=I57-5wzAwlTEb5_opfZgP1ZltaM
- Organization WH. High burden to high impact: a targeted malaria response [Internet]. World Health Organization; 2018 [cited 2024 Aug 2]. Available from: https://apps.who.int/iris/bitstream/handle/10665/275868/WHO-CDS-GMP-2018.25-eng.pdf
- World Health Organisation. World Malaria Report, 2021.
- Namusoke F, Rasti N, Kironde F, Wahlgren M, Mirembe F. Malaria Burden in Pregnancy at Mulago National Referral Hospital in Kampala, Uganda. Malar Res Treat. 2010 Nov 7;2010:1–10.
- Okiring J, Olwoch P, Kakuru A, Okou J, Ochokoru H, Ochieng TA, et al. Household and maternal risk factors for malaria in pregnancy in a highly endemic area of Uganda: a prospective cohort study. Malar J. 2019 Dec;18(1):144.
- Lack of effect of intermittent preventive treatment for malaria in pregnancy and intense drug resistance in western Uganda | Malaria Journal [Internet]. [cited 2024 Aug 3]. Available from: https://link.springer.com/article/10.1186/s12936-015-0909-7?error=cookies_not_supported&code=5ddc31ed-26bb-42fe-9c07-fb0bf1a8a4e1
- Prevalence and risk factors for Plasmodium falciparum malaria in pregnant women attending antenatal clinic in Bobo-Dioulasso (Burkina Faso) | BMC Infectious Diseases [Internet]. [cited 2024 Aug 3]. Available from: https://link.springer.com/article/10.1186/s12879-014-0631-z?error=cookies_not_supported&code=0c833537-9ebe-4b4f-b748-b82836a5d063
- Prevalence and risk factors associated with malaria infection among pregnant women in a semi-urban community of north-western Nigeria | Infectious Diseases of Poverty [Internet]. [cited 2024 Aug 3]. Available from: https://link.springer.com/article/10.1186/s40249-015-0054-0?error=cookies_not_supported&code=032795c6-4134-43b6-b248-73c8dda78217
- Impact of malaria during pregnancy on pregnancy outcomes in a Ugandan prospectivecohort with intensive malaria screening and prompt treatment | Malaria Journal [Internet]. [cited 2024 Aug 3]. Available from: https://link.springer.com/article/10.1186/1475-2875-12-139?error=cookies_not_supported&code=2e6bd2de-1b22-4397-8071-1883d9a72178
- Non-adherence to long-lasting insecticide treated bednet use following successful malaria control in Tororo, Uganda | PLOS ONE [Internet]. [cited 2024 Aug 3]. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0243303
- MOH, Uganda. MInistry of Health, Uganda Malaria Reduction Strategic Plan 2014–2020. 2014, Ministry of Health: Plot 6, Lourdel Road Nakasero.
- Agyeman YN, Newton S, Annor RB, Owusu-Dabo E. Intermittent preventive treatment comparing two versus three doses of sulphadoxine pyrimethamine (IPTp-SP) in the prevention of anaemia in pregnancy in Ghana: A cross-sectional study. PLoS One. 2021;16(4):e0250350.
- Orish VN, Onyeabor OS, Boampong JN, Afoakwah R, Nwaefuna E, Acquah S, et al. Prevalence of intermittent preventive treatment with sulphadoxine-pyrimethamine (IPTp-SP) use during pregnancy and other associated factors in Sekondi-Takoradi, Ghana. Afr Health Sci. 2015;15(4):1087–96.
- Epstein A, Maiteki-Sebuguzi C, Namuganga JF, Nankabirwa JI, Gonahasa S, Opigo J, et al. Resurgence of malaria in Uganda despite sustained indoor residual spraying and repeated long lasting insecticidal net distributions. PLOS Glob Public Health. 2022;2(9):e0000676.
- WHO. World Malaria Report, 2022.
- Uganda Bureau of Statistics (UBOS) and ICF Kampala and Rockville: UBOS and ICF International. Uganda malaria indicator survey report; 2018–19.
- Uganda National Malaria Control Division. National Malaria Control Division Mid Term Review 2022.
- DHI. District Health Information System-2 (DHIS2).
- Rogerson SJ, Mhango CG, Molyneux ME, Qongwane C, Chaluluka E, Van Den Broek NR. Malaria and anemia in antenatal women in Blantyre, Malawi: a twelve-month survey. Am J Trop Med Hyg. 2000 Mar 1;62(3):335–40.
- atient perspectives on interpersonal aspects of healthcare and patient-centeredness at primary health facilities: A mixed methods study in rural Eastern Uganda | PLOS ONE [Internet]. [cited 2024 Aug 5]. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0236524


Comments are closed.