Trends and distribution of Intermittent Preventive Treatment utilization for malaria, pregnancy, Uganda, 2017-2022
Authors: Daniel Orit1*, Benon Kwesiga1, Alex R. Ario1, Richard Migisha1, Jane Nabakooza2, Hildah Tendo Nansikombi1, Gerald Rukundo2; Institutional affiliations: 1Uganda Public Health Fellowship Program, Uganda National Institute of Public Health, Kampala, Uganda, 2National Malaria Control division, Ministry of Health, Uganda; Correspondence*: Tel: +256777322263, Email: dorit@uniph.go.ug
Summary
Background: sulfadoxine-pyrimethamine’In Uganda, malaria in pregnancy prevalence ranges from 8.9% in low transmission arears to >50% in high transmission area, and in 2019, 152 (4.3%) of the 3,528 maternal death were due to malaria in pregnancy. At least 3 doses of sulfadoxine-pyrimethamine’ (IPTp-SP3) given during antenatal care (ANC) is expected to prevent malaria in pregnancy, reduces its negative effects, and additionally reduce cases of malaria in pregnancy. As of 2015, the recommended target for IPTp-SP3 utilization in Uganda is ≥ 66%utilization. Despite significant progress in ANC attendance, the extent of IPTp-SP utilization in Uganda remains unknown. We determined the trends in IPTp-SP3 utilization among ANC attendees and its relationship with malaria in pregnancy (MIP) during 2017–2022.
Methods: We extracted data on IPTp-SP3, ANC attendance (ANC1 and MIP from the District Health Information System 2 (DHIS2), 2017–2022. We calculated the national, regional, and district IPTp-SP3 utilization as the proportion of pregnant women (number who had attended ANC1) who received at least 3 doses of IPTp-SP3 during 2017–2022. MIP was calculated as a proportion of pregnant with malaria who attended ANC1. Mann-Kendall test was used to evaluate significance of linear trends.
Results: Despite the overall IPTp-SP3 utilization increasing from 2.5% to 50% during 2017–2022, it was below the recommended target of ≥ 66%. Regions of South Buganda (-33%), Karamoja (-19%), and Bukedi (-13%) experienced a decreasing trend in IPTp-SP3 utilization during the period 2017–2022, while only Acholi (+30) and Toro (+27%) regions had an increasing trend in IPTp-SP3 utilization during the period 2017–2022. Malaria in pregnancy cases increased from 12% to 22% during the period 2017–2022, despite a rising trend in percentage IPTp3 utilization across the period.
Conclusion: There was suboptimal IPTp-SP3 utilization in Uganda from 2017–2022, with the target ≥ 66% utilization not achieved. Declining trends in IPTp-SP3 utilization may result to an increase in malaria in pregnancy. Further investigation into reasons for low uptake of IPTp-SP3 among pregnant women, particularly in regions with declining IPTp-SP3 utilization could provide insights to help improve uptake.
Background
Malaria remains a major public health concern, especially among pregnant women and children under five years of age (1, 2). Over 30 million women in Africa become pregnant in malaria-endemic areas, where they are at higher risk of malaria infection compared to non-pregnant women (1, 2). Malaria in pregnancy (MIP) is often associated with unfavorable outcomes, such as stillbirth, low birthweight, pre-term delivery, abortion, and maternal death (1, 2). According to the Uganda Malaria Indicator Survey 2015, malaria among pregnant women accounts for about 14% of outpatient services utilization, 11% of admissions, and 9% of deaths (3)). This is critical considering that key strategic interventions exist to prevent and control malaria transmission during pregnancy (3),
To reduce morbidity and mortality associated with malaria, the World Health Organization (WHO) recommended the use of the Intermittent Preventive Treatment for malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP) in 2000, alongside other preventive and curative interventions. Intermittent Preventive Treatment for malaria in pregnancy using 3 doses of sulfadoxine-pyrimethamine protects between 65% and 85% of pregnant women from placental malaria infection (4, 5, 13). Subsequently, the Uganda National Malaria Control Division (NMCD) revised the national IPTp-SP policy from two doses of IPTp-SP as a directly observed treatment strategy during routine antenatal care (ANC) to three or more doses of IPTp-SP3 during ANC (5). The three or more IPTp-SP doses are linked to a greater mean birth weight and fewer low birth weight (LBW) newborns than the two doses (12). Additionally, the NMCD revised the national target for IPTp-SP3 uptake from 85% to ≥ 66% IPTp-SP3 utilization.
Although pregnant women in many districts of Uganda (16) have registered progress in ANC attendance (65% in 2013 to 90% in 2022), the extent of IPTp-SP3 utilization remains unknown in Uganda. We determined the trends in utilization of IPTp-SP3 among ANC attendant’s registrants and its relationship with malaria in pregnancy in Uganda, 2017-2022.
Methods
Study setting
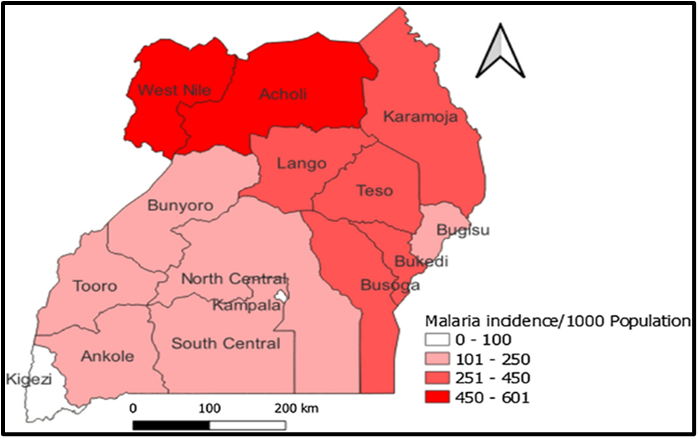
Uganda comprises 15 health regions, of which two (West Nile and Acholi Regions) are considered areas with high annual malaria transmission rates (annual parasite index (API) >450 cases/1,000 population), five (Lango, Karamoja, Teso, Bukedi, and Busoga Regions) are considered medium malaria transmission areas (API=251-450 cases/1,000 population), and six (South Central, North Central, Kampala, Ankole, Tooro, Bugisu, and Bunyoro Regions) are considered low malaria transmission areas (API=101-250 cases/1,000 population). Kigezi Region is considered to have very low malaria transmission, with API≤100 cases/1,000 population/year (Figure 1)and is targeted for malaria elimination in the Uganda National Malaria Strategic Plan 2025 (14,15,16).
Study design, data source, and variables
We performed a descriptive analysis of routinely reported surveillance aggregated data. These data are summarized at health facility level using the Health management Information System, form 105 and then entered in the national electronic District Health Information System version 2 (DHIS2) [7]. The HMIS Form 105 is a report on outpatient department (OPD) attendances, diagnoses, maternal and child health (MCH), HIV/AIDS service, laboratory and stock status of essential drugs and supplies, among others [7], which is compiled by the health facility and submitted to MoH through the DHIS 2 at the end of every month. We extracted data on total monthly ANC attendance, total monthly first ANC, total monthly 4+ANC attendance, total monthly IPTp-SP3 utilization, and annual antenatal clinic malaria in pregnancy cases. In addition, we extracted data on HMIS 105 reporting rates.
Data analysis
Intermittent prevention treatment for malaria in pregnancy utilization was computed using the number of ANC registrants who received at least 3 doses of IPTp-SP3 by the total number of ANC1 attendants. First antenatal care attendance is a proxy indicator, the WHO recommends that ANC1 is the standard denominator used when computing IPTp-SP3 utilization. The assumption made is that all pregnant women who initiate ANC1 will at least attend 3 or more ANC visits by the time they reach term pregnancy for delivery. The midwives have been empowered to provide adequate knowledge and encouragement to women attending ANC 1 such that they are motivated to attended subsequent visits.
Malaria in Pregnancy (MIP) was computed as the proportion of pregnant women attending ANC1 who tested positive for malaria. We generated national and regional temporal trends for the period 2017–2022. We compared IPTp-SP3 trends with malaria in pregnancy trends through the 6-year period 2017-2022. We used line graphs to demonstrate the trends and Mann-Kendall test to determine the statistical significance of the trends. Data was analyzed using STATA version 14.
Ethical considerations
The Ministry of Health Uganda provided administrative clearance to conduct this investigation. In addition, we received a non-research determination clearance from the US Centers for Disease Prevention and Control (US CDC). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy. § §See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq. No informed consent from participants was sought since secondary data was used.
Results
Trend of intermittent prevention treatment utilization for malaria in pregnancy among pregnant women attending antenatal care, Uganda, 2017 – 2022
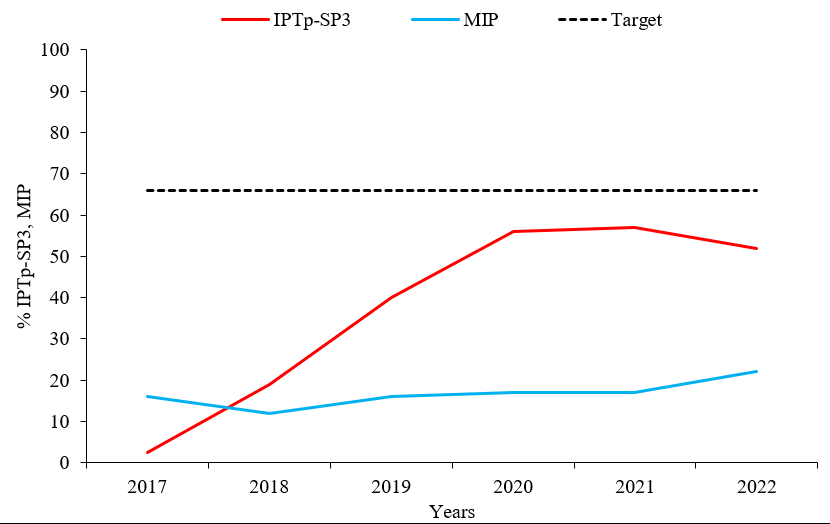
Intermittent prevention treatment utilization for malaria in pregnancy (IPTp-SP3) by pregnant women attending antenatal care in Uganda during 2017–2022 was 2.5% in 2017 and 50% in 2022. A significant decline in IPTp-SP3 utilization from 57% in 2021 to 50% (p= 0.048) in 2022 was registered. The national target for % IPTp-SP3 utilization was below the 66% target for the 6-year period 2017 through 2022. Malaria in pregnancy (MIP) cases increased from 12% to 22% (p=0.05) during the period 2017-2022 despite a rising trend in % IPTp-SP3 utilization of 2.5-50% (p=0.048) across the annual periods 2017-2022. Additionally, malaria in pregnancy was high in 2017 compared to a low IPTp-SP3 utilization in 2017 (Figure 1).
Intermittent prevention treatment for malaria in pregnancy utilization by districts, Uganda, 2017-2022
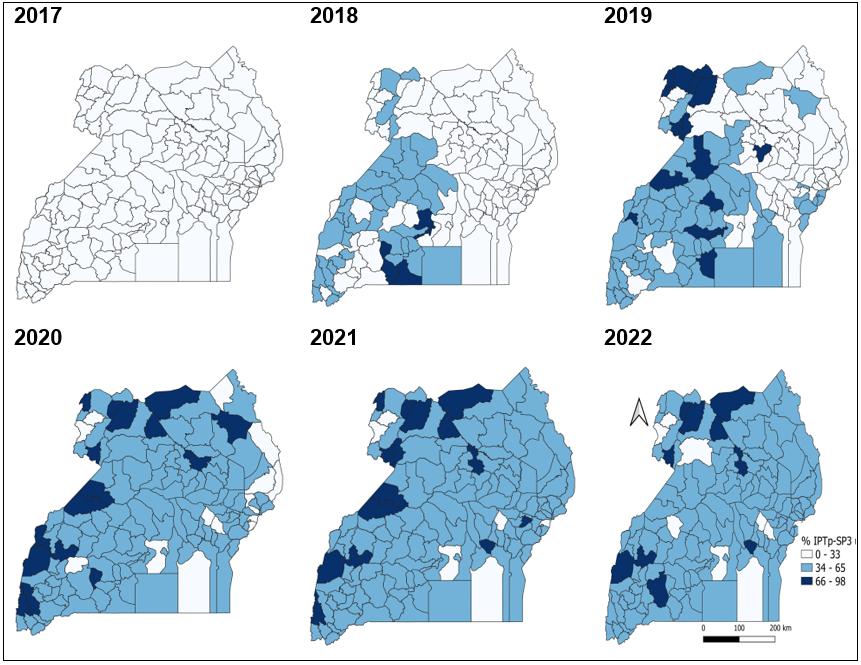
There was an increasing coverage in IPTp-SP3 utilization during 2017-2022, with the districts of Lamwo, Gulu, Adjumani, Obongi, Kasese, and Kamwenge having a consistently high (>66%) IPTp-SP3 utilization during 2020-2022. Districts of Buvuma, Mpigi, Bududa, Kaliro, and Namutumba continued to have a consistently low IPTp-SP3 utilization across the 6-year study period (Figure 2)
Discussion
Despite the overall IPTp-SP3 utilization increasing from 2.5% to 50%, the set target of 66% was not attained. There was an increasing trend in MIP during the 6-year study period. Malaria in pregnancy prevalence was highest in 2017 and 2018, a period when IPTp-SP3 utilization was lowest. Three regions, South Buganda, Karamoja , Bukedi region experienced a decreasing trend in IPTp-SP3 utilization, while two regions Acholi region,Toro region, had an increasing trend in IPTp-SP3 utilization during the period 2017–2022.
Our results indicate a gradual suboptimal improvement in IPTp-SP3 utilization in majority districts from 2017-2022, which supports similar study findings in Ghana, Tanzania (10, 12). This improvement is likely due to adjustments in the policy made by the WHO on antenatal care visits by pregnant women from at least four ANC visits to more than eight ANC visits before giving birth. Having more ANC visits ensures that pregnant women at least initiate three doses of IPTp-SP3 during pregnancy (11). In spite of the observed suboptimal improvements in IPTp-SP3 utilization in majority districts, the overall utilization remains insufficient below the recommended 66% national target, and varies greatly by districts.
Districts located in the island (Buvuma and Kalangala) consistently reported low IPTp-SP3 utilization throughout the 6-year period 2017-2022. Our findings are consistent with studies conducted in the African setting with similar malaria endemicity (8, 10, and 11). Although, Basha GW et al (11) argued that challenges of low IPTp-SP3 utilization data seem to revolve around attitudes and perceptions by pregnant women in Ethiopia, this may not be the case in some districts in Uganda. Low IPTp-SP3 utilization might be influenced by contextual factors such as, poor motivation of health workers at the antenatal clinics, challenges with access to the antenatal clinics, cultural practices about medication given during pregnancy, frequent stock outs of drugs used for IPTp-SP3, and inadequate human resource at the health facilities (8, 10). In addition, the COVID-19 pandemic in 2020-2021 affected access of pregnant women to health facilities for antenatal care (21). This finding emphasizes the need for context-specific interventions especially in antenatal clinics. Additionally, there is need to further explore these contextual factors.
We note a small but significant increasing trend in malaria in pregnancy cases during the study period despite a rising trend in % IPTp-SP3 utilization across the period. The increasing trend of malaria cases among pregnant women attending antenatal care as found in the current study is consistent with findings in Malawi, Ghana (16,17). However, our findings contrasts with findings in Kenya (19, 20), where malaria in pregnancy was low in concordance with a high % IPTp-SP3 utilization, possibly due to improved health systems in Kenya where great emphasis is placed on maternal child health indicators (19). The rising malaria in pregnancy cases amidst IPTp-SP3 utilization could be attributed to increasing resistance of malaria parasites to sulfadoxine-pyrimethamine antimalarial. (18). Additionally, our current study did not demonstrate the beneficial effect of higher doses IPTp-SP being able to protect pregnant women against episodes of malaria, possibly because of increasing resistance to sulphadoxine-pyrimethamine, the drug used in IPTp-SP (18).
Study limitations
The study has some limitations that should be considered when interpreting the results. We used secondary data from the District Health Information System 2 (DHIS2), which has challenges of completeness and accuracy, likely leading to either over or under estimation of the IPTp-SP3 utilization among pregnant women and malaria in pregnancy cases.
Conclusion
Utilization of the recommended three or more doses of IPTp-SP remains low. Most pregnant women are less likely to take the recommended three or more doses of IPTp-SP. Malaria in pregnancy cases are still high despite improved IPTp-SP3 utilization.
We recommended further studies to assess the factors associated with low utilization of IPTp-SP3, and resistance patterns to sulphadoxine-pyrimethamine in Uganda.
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ Contributions
OD conceptualized the idea, analyzed and interpreted the data and drafted the manuscript. BK, ARA, RM, JN, GR, and HTN critically reviewed the bulletin for intellectual content.
Acknowledgements
The authors appreciate the Ministry of Health for providing access to DHIS2 data that was used for this analysis. We further appreciate the National Malaria Control Division and other national malaria stakeholders for raising the questions that initiated this analysis.
Copyright and licensing
All material in the UgandaPublic Health Bulletin is in the public domain and may be used and reprinted without permission. However, citation as to source is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- World Health Organization (WHO). WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP. Geneva: World Health Organization, 2013.
- World Health Organization (WHO). WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP. Geneva: World Health Organization, 2013.
- Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007;7:93–104.
- Garner P, Gulmezoglu A,et al. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;4.
- Mbonye AK, Bygbjerg I, Magnussen P. Intermittent preventive treatment of malaria in pregnancy: a community-based delivery system and its effect on parasitemia, anemia and low birth weight in Uganda. Int J Infect Dis 2008;12:22–9.
- JHPIEGO. Community Intermittent Preventive Treatment for Malaria in Pregnancy Implementation Guide Version 2, 2018.
- Arthur E. Wealth and antenatal care use: implications for maternal health care utilisation in Ghana. Health Econ Rev 2012;2:14.
- Andrew EV, Pell C, Angwin A, et al. Factors affecting attendance at and timing of formal antenatal care: results from a qualitative study in Madang, Papua New Guinea. PLoS One 2014;9:e93025.
- Ouma PO, Van Eijk AM, Hamel MJ, et al. The effect of health care worker training on the use of intermittent preventive treatment for malaria in pregnancy in rural western Kenya. Trop Med Int Health 2007;12:953–61.
- Marchant T, Nathan R, Jones C, et al. Individual, facility and policy level influences on national coverage estimates for intermittent preventive treatment of malaria in pregnancy in Tanzania. Malar J 2008;7:260.
- Basha GW. Factors affecting the utilization of a minimum of four antenatal Care Services in Ethiopia. Obstet Gynecol Int. 2019. Accessed September 15, 2020. DOI: https://doi.org/10.1155/2019/5036783
12.Addai-Mensah O, Annani-Akollor ME, Fondjo LA, et al. Regular antenatal attendance and education influence the uptake of intermittent preventive treatment of malaria in pregnancy: a cross-sectional study at the university hospital, Kumasi, Ghana. J Tropic Medic. 2018; 2018: 5019215. DOI: https://doi. org/10.1155/2018/5019215
- Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev. 2006;4:CD000169.
- McKelvie, W.R., A.A. Haghdoost, and A. Raeisi, Defining and detecting malaria epidemics in south-east Iran. Malaria journal, 2012. 11(1): p. 1-8.
- National Malaria Control Program, M.o.H.U., Guidelines for Prevention, Preparedness and Response for Malaria Epidemics. 2019. p. 19-20.
- Division of Health Information, M.o.H.U., District health Information system. 2022.
- Anchang-Kimbi JK, Achidi EA, Apinjoh TO, Mugri RN, Chi HF, Tata RB, et al. Antenatal care visit attendance, intermittent preventive treatment during pregnancy (IPTp) and malaria parasitaemia at delivery. Malaria Journal. 2014;13(1):1–9. doi: 10.1186/1475-2875-13-162.
- Nkoka O, Chuang T-W, Chen Y-H. Association between timing and number of antenatal care visits on uptake of intermittent preventive treatment for malaria during pregnancy among Malawian women. Malaria journal. 2018;17(1):1
- Desai M, Gutman J, L’Lanziva A, Otieno K, Juma E, Kariuki S, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet. 2015;386(10012):2507–19. pmid:26429700.
- Kakuru A, Jagannathan P, Muhindo MK, Natureeba P, Awori P, Nakalembe M, et al. Dihydroartemisinin-Piperaquine for the Prevention of Malaria in Pregnancy. N Engl J Med. 2016;374(10):928–39.
- JHPIEGO. Community Intermittent Preventive Treatment for Malaria in Pregnancy Implementation Guide Version 6, 2023.


Comments are closed.