Multiple sporadic Crimean-Congo Haemorrhagic Fever Outbreaks, Uganda, July 2018-January 2019
Author: Bernadette Basuta Mirembe1 Angella Musewa1 Esther Kisaakye1 Doreen Birungi1 Luke Nyakarahuka2 Stephen Balinandi2 Alex Tumusiime2 Jackson Kyondo2 Sophie Mulei2 Jimmy Baluku2 Daniel Kadobera1 Benon Kwesiga1 Steven N Kabwama1 Alex Ri-olexus Ario1; Affiliations: 1Uganda Public Health Fellowship Program, Kampala, Uganda 2Uganda Virus Research Institute, Entebbe, Uganda
Summary
In 2018, the Ministry of Health confirmed multiple sporadic CCHF outbreaks between July 2018 and January 2019. The sporadic outbreaks affected 11 districts. We conducted investigations to determine the scope of these outbreaks, identify risk factors for evidence-based interventions. We identified 14 case-patients from 11 districts located in Western and Central Uganda. The case fatality was 36% (5/14). The males (AR 4.4/1,000,000) were more affected than females (AR/1,000,000). 71% (10/14) of the case-patients had found ticks attached to their bodies compared to 27% (15/56) of the control-persons (AOR 9.3, 95%CI 1.9-46). The CCHF out-break was associated to tick exposure. We recommend strengthening of tick control strategies and regulation of animal movements to reduce the dispersal of the infection.
Background
Crimean-Congo Haemorrhagic Fever (CCHF) is a zoonotic viral haemorrhagic fever caused by a tick-borne virus. The primary form of transmission of the virus between animals and humans is through ticks though contact with infectious blood or body fluids can lead to infection [1]. The incuba-tion period for CCHF is 1-9 days following a tick bite and 5-13 days through contact with infectious blood or body fluUganda has had 9 CCHF confirmed outbreaks since 2013. The disease occurred and was detected more in 2018 compared to previous years. The first confirmed outbreak was in 2013 in Agago District with 3 cases, 2013 Wakiso (2 cases), 2015 Nak-aseke (1 case), 2017 Kiboga (1 case), 2017 Nakaseke (1 Case), and 2017 Luweero (1 case) and early 2018, outbreaks were con-firmed in Nakaseke and Mubende districts.
In July 2018, Uganda Virus Research Institute (UVRI) con-firmed 2 CCHF case-patients by PCR. Subsequently for 7 months, suspected CCHF case-patients were sporadically re-ported and confirmed by PCR. We conducted investigations to determine the scope of these outbreaks, and identify risk factors for evidence-based interventions.
Methods
We defined a suspected CCHF case as sudden onset of fever (>380 C) with any four of the following symptoms; loss of ap-petite, vomiting, diarrhoea, headache, abdominal pain, joint pain and sudden onset of unexplained bleeding in a resident in Isingiro, Kiryandongo, Luweero, Mukono, Nakaseke, Rakai, Ibanda, Kabarole, Rukungiri, Masindi, and Kiruhura districts from 1 July 2018 – 30 January 2019. We defined a confirmed case as suspected case positive for CCHF by Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) assay or Immunoglobulin (IgM) serology.
We used the UVRI surveillance data to identify case-patients coupled with review of hospital records. We described case-persons by: person, place, time, and symptom characteristics. We interviewed 8 cases and generated a hypothesis that we tested in a case-control study. We hypothesized that case-patients were associated with presence of tick-infested animals in their household or neighbourhood. Using all case-patients; each compared to 4 control-persons matched by age, sex, and village, we tested the hypothesis through interviews guided by a structured questionnaire.
Findings
Descriptive epidemiology and case-control study findings
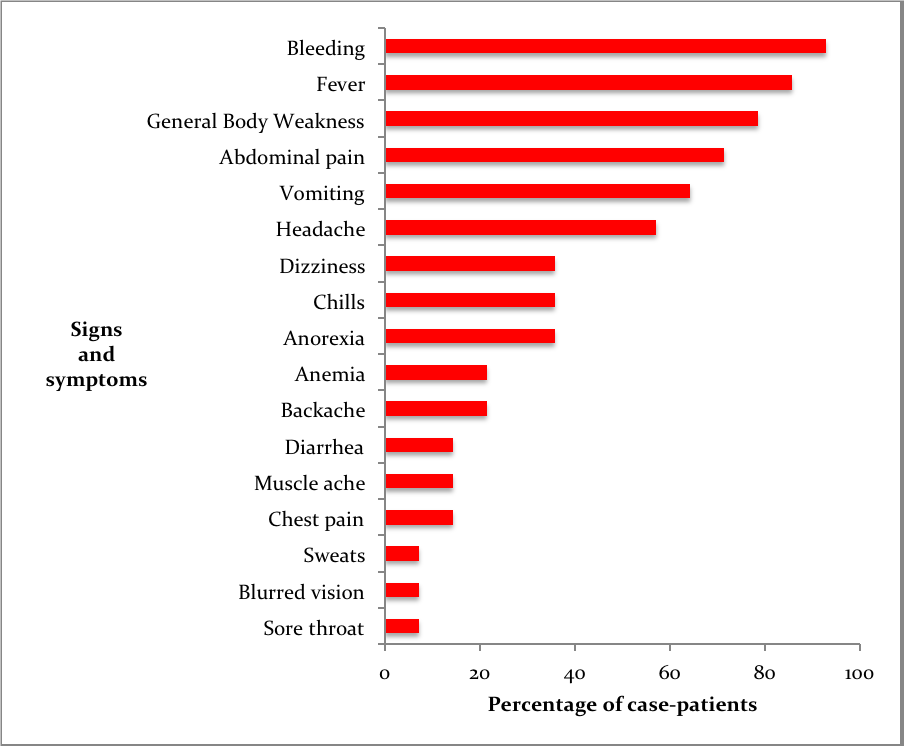
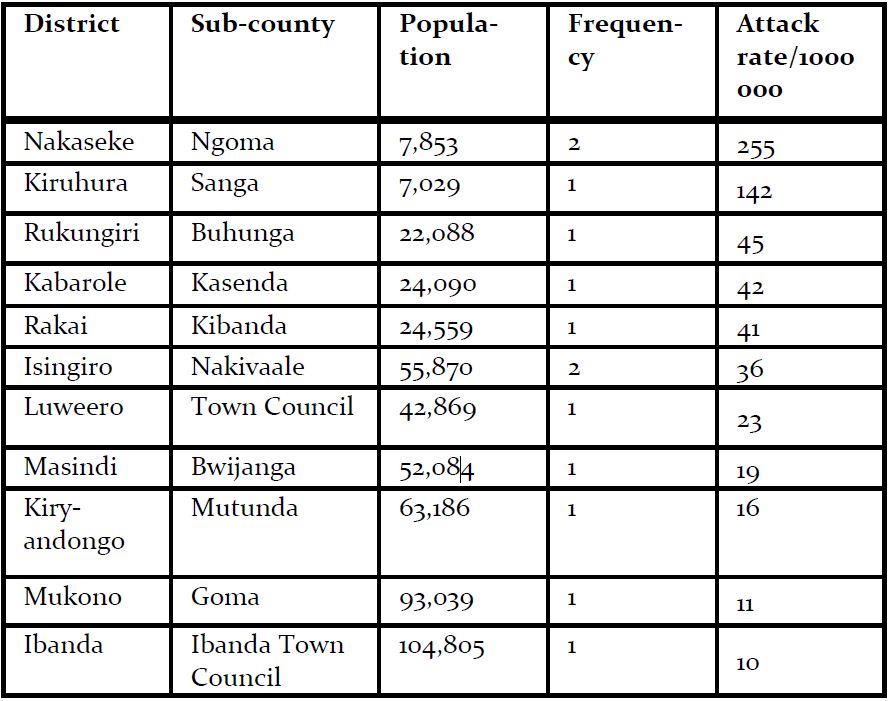
Fourteen confirmed case-patients were line listed between July 2018 and January 2019 from 11 districts, of which 5 died (case fatality rate=36% (5/14) The mean age of case-patients was 24 (SD±9). Most case-patients were aged between 19-35 years of age. 93% (13/14) of the case patients experienced bleeding and fever (Figure 2). Of the case-patients, 64% (9/14) were males whereas 36% (5/14) were females. Males (AR 4.4/1,000,000) were more affected by females (AR 2.4/1000000). The case-patients lived in Isingiro (2), Mukono (1), Nakaseke (2), Kiryandongo (2), Luweero (1), Rakai (1), Ibanda (1), Kabarole (1), Rukungiri (1), Masindi (1) and Kiruhura (1), all located in Central and Western Uganda. Most of the districts (73%) affected have a high cattle population (>140,000 cattle) according to the 2010 animal census (Figure 1). Nakaseke District was the most affected district (AR 10/1000000) where as Mukono District was the least affected (AR 1.7/1,000,000). Ngoma sub-county in Nakaseke District was the most affected (AR 255/1,000,000) where as Ibanda Town council was the least affected (10/1,000,000) (Table 1).

In terms of risk factors, 71% of the case-patients had found ticks attached to their bodies compared to 27% of the control-persons (AOR 9.3, 95%CI 1.9-46).
Discussion
Our study found case-patients had higher odds of having tick exposure than control-persons. This was similar to prior studies carried out during CCHF outbreaks in Uganda that linked case-patients to tick exposure. In 2017, Kizito et al found case-patients had higher odds of having had tick exposure (OR 11, 95%CI 1.1-112) [3] than control-persons. Additionally, Balinandi et al had linked the isolated CCHF case-patient in 2015 to tick exposure after isolating CCHFv RNA by RT-PCR from one of the tick samples picked from animals proximal to the case-patient [4].
CCHF outbreaks in Uganda are on a rise. From 2013, less than 10 outbreaks have been confirmed however we observe 13 sporadic outbreaks occurring within 7 months between 2018 and 2019. We could relate this observation to the VHF surveillance program that has effectively detected VHF outbreaks robustly [5]. Alternatively, it could be due to the increase in the suspicion index of health workers due to the Ebola outbreak in Democratic Republic of Congo (DRC) hence increased surveil-lance [6]. However, widespread tick resistance that is burdening this country could also be linked to rise in occurrence of these outbreaks.
No definitive preventive and control strategies have been widely implemented successful due to little understanding of the pathogenesis of the disease [1]. Antibodies in livestock are the best indicator of risk of CCHF in humans. Such serological studies have not been adequately explored in Uganda thus risk in humans has not been fully assessed. However, ticks being the known vectors transmitting the disease, tick control is paramount in preventing and controlling future outbreaks.
Limitations
Due to logistical limitations, we were unable to confirm presence of infected animals around the case-patients. At the time of the investigation, there was an ongoing Ebola outbreak in the DRC that resulted in an overwhelming number of suspect-ed EVD samples at the UVRI VHF laboratory. We were there-fore unable to test animal and tick samples collected during the investigation due to shift of priority towards intensified Ebola screening.
Conclusions and recommendations
The CCHF outbreaks were sporadic with 14 confirmed case-patients from 11 districts within 7 months period. The out-breaks were associated to tick exposure. Animal movements might have led to the dispersion of the outbreaks. We recommend that Ministry of Agriculture, Animal Industry and Fisheries (MAAIF) should strengthen tick control strategies and regulate animal movements to reduce the dispersal of the infection.
References
1. Al-Abri SS, Al Abaidani I, Fazlalipour M, Mostafavi E, Leblebicioglu H, Pshenichnaya N, et al. Current status of Crimean-Congo haemorrhagic fever in the World Health Organization Eastern Mediterranean Region: issues, challenges, and future directions. International Journal of Infectious Diseases. 2017;58:82-9.
2. Appannanavar SB, Mishra B. An Update on Crimean Con-go Hemorrhagic Fever. J Glob Infect Dis. 2011;3(3):285-92. doi: 10.4103/0974-777x.83537. PubMed PMID: 21887063; PubMed Central PMCID: PMCPmc3162818.
3. Kizito S, Okello PE, Kwesiga B, Nyakarahuka L, Balinandi S, Mulei S, et al. Notes from the Field: Crimean-Congo Hemorrhagic Fever Outbreak – Central Uganda, August-September 2017. MMWR Morbidity and mortality weekly report. 2018;67(22):646-7. Epub 2018/06/08. doi: 10.15585/mmwr.mm6722a6. PubMed PMID: 29879093; PubMed Central PMCID: PMCPMC5991810.
4. Balinandi S, Patel K, Ojwang J, Kyondo J, Mulei S, Tu-musiime A, et al. Investigation of an isolated case of human Cri-mean-Congo hemorrhagic fever in Central Uganda, 2015. Inter-national journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2018;68:88-93. Epub 2018/02/01. doi: 10.1016/j.ijid.2018.01.013. PubMed PMID: 29382607; PubMed Central PMCID: PMCPMC5893389.
5. Shoemaker TR, Balinandi S, Tumusiime A, Nyakarahuka L, Lutwama J, Mbidde E, et al. Impact of enhanced viral haemorrhagic fever surveillance on outbreak detection and response in Uganda. The Lancet Infectious Diseases. 2018;18(4):373-5.
6. Gostin L, Phelan A, Coutinho AG, Eccleston-Turner M, Erondu N, Filani O, et al. Ebola in the Democratic Republic of the Congo: time to sound a global alert? The Lancet. 2019.


Comments are closed.