Measles outbreak with children below the recommended age for first dose of measles vaccine most affected in Kakumiro District, February–May 2024
Authors: Emmanuel Okello Okiror*1,2, Immaculate Ampaire2, Fred Nsubuga2, Joanita Nalwanga1, Patrick Kwizera1, Paul Edward Okello1, Richard Migisha1, Benon Kwesiga1, Alex Riolexus Ario1; Institution affiliation: 1Uganda Public Health Fellowship Program, Uganda National Institute of Public Health, Kampala, Uganda; 2Uganda Expanded Program on Immunization, Ministry of Health Uganda, Kampala, Uganda; Correspondence*: Tel: +256 776 353542, Email: okiroreo@uniph.go.ug
Summary
Background: On April 7, 2024, the Uganda Ministry of Health was notified of a measles outbreak in Kakumiro District involving death of a suspected case. We investigated to determine the scope of the outbreak, assess risk factors for disease transmission, and recommend evidenced-based control and prevention interventions.
Methods: We defined a suspected case as onset of fever and maculopapular generalized rash with ≥1 of cough, coryza, or conjunctivitis in any resident of Kakumiro District from February 1–May 30, 2024. A confirmed case was a suspected case with laboratory confirmation for measles Immunoglobulin M (IgM) antibody. We conducted active case search at health facilities and communities to line-list suspected case-patients. We conducted unmatched case-control to identify factors associated with measles transmission. We identified risk factors using conditional logistic regression. We inspected health centers, trading centers and households to further identify factors that facilitated the spread of measles. We estimated vaccine coverage and Vaccine Efficacy.
Results: We identified 188 suspected cases, including 6 (3.2%) confirmed and 1 (0.5%) death. The overall attack rate (AR) was 67/100,000 persons. Children aged <9 months (AR=232/100,000) and those aged 9m–≤5 years (AR=177/100,000) were the most affected. The most affected subcounties were Kisengwe (AR=313/100,000), Kasambya (AR=126/100,000) and Kakumiro town council (AR=110/100,00). Non-vaccination (aOR=2.9, 95%CI: 1.1-7.6), exposure to a measles case-patient in a crowded health facility during exposure period (aOR=47, 95%CI: 6.09-369), and exposure to measles case-patient in the same household during exposure period (aOR=9.3, 95%CI: 2.9-30) were associated with measles infections. Vaccine coverage was 88%
(95%CI: 79%-94%) and vaccine efficacy was 65% (95%CI: 13%-91%). We observed crowdedness and lack of triaging/isolation in health facilities.
Conclusions: This outbreak was facilitated by non-vaccination and propagated by exposure to infected persons in crowded health facilities and households. We recommended to MoH to conduct a supplementary immunization activity that included children <9 months in the target group. Triaging and isolation of case-persons might help to reduce the spread of measles in future outbreaks. There is also need to develop strategies to improve vaccine efficacy in the district.
Background
Measles remains a global public health challenge with an estimated 136,000 people dying from it in 2022 alone (1). Case fatality rates (CFRs) of measles range from 0.1% in developed countries to 15% in developing countries; and are highest among unvaccinated children under 5 years and lowest among vaccinated children regardless of their setting (1, 2). In 2019, data from the World Health Organization (WHO) showed that measles cases had risen by 300% globally and 700% in the African region (3). The disproportionate burden in Africa is compounded by influx of refugees, inadequate funds for immunization activities and reduced vaccine efficacy due to poor vaccine management and injection safety measures (4).
Vaccination is one of the most effective ways to prevent measles and its spread in populations. It is estimated that vaccination successfully averted globally, an estimated 57 million deaths between 2000–2022 and decreased measles deaths from an estimated 761,000 in 2000 to 136,000 in 2022 (5). Uganda offers 2 doses of measles-rubella (MR) as recommended by the WHO guidelines (1). The first dose (MR1) which was introduced in 1981 is given at 9 months and the second dose (MR2) only recently introduced in 2022 is given at 18 months. There was a noted improvement in the vaccine coverage (VC) for the single dose of measles containing vaccine in a 10 years period from 83% in 2013 to 93% in 2023 (6). The second dose on the contrary had a decrease in VC from 49% in 2022 to 21% in 2023 (6). To achieve herd immunity sufficient to prevent outbreaks, the WHO recommends a vaccination coverage of 95% for any antigen (1). However, the global vaccination coverage for the MR1 still stagnates at 86% and that of MR2 at 70%. Similarly, in Uganda, these vaccine coverages are lower than the recommended with MR1 coverage at 90% and MR2 at only 28% as of the third quarter financial year 2023/2024 (DHIS2 data). As of mid-year, 2024, eighteen districts countrywide had reported and confirmed measles outbreaks according to the Uganda Expanded Program on Immunization (UNEPI) third quarter, 2023/2024 financial year report. The outbreak in Kakumiro was notified to the Ministry of Health on April 7, 2024.
We investigated this outbreak to determine its scope, assess risk factors for disease transmission, assess vaccine coverage and effectiveness, and recommend evidenced-based control and prevention interventions.
Methods
Outbreak area
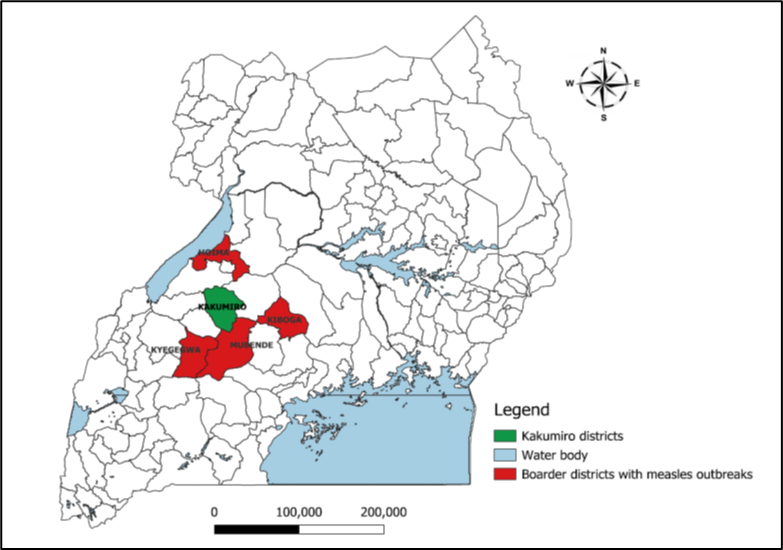
Kakumiro District is located in the western region of Uganda. Administratively, Kakumiro constitutes of 24 sub-counties with a population of approximately 651,200 people. About 333,200 are males and 318,000 females as of 2024. The MR vaccination coverage in, Kakumiro prior to the outbreak was 61% for MR1 and only 10% for MR2 (DHIS2, January 2024). Four of the five districts bordering Kakumiro had prior reported and responded to measles outbreaks including Hoima, Kyegegwa, Kiboga and Mubende districts (Figure 1).
Case definition and finding
We defined a suspected case as onset of fever and maculopapular generalized rash with one or more of the following: cough, coryza or conjunctivitis in any resident of Kakumiro District from February 1 to May 30, 2024. A confirmed case was a suspected case with laboratory confirmation for measles IgM antibodies
We conducted active case search in both health facilities and communities. In health facilities, we reviewed health records including out-patient department (OPD) and in-patient department (IPD) registers. In the communities, with the help of village health teams (VHTs) and health assistants, we conducted house to house search and also found cases through snow balling. All the case-patients identified from both health facility and community active case searches were line listed and this was updated daily.
Descriptive epidemiology
We calculated the attack rates by person (age, sex) and place (subcounty) using projected population estimates from Uganda Bureau of Statistics (UBOS, 2024) as denominators per 100,000 persons (7).
We used an epicurve to assess the distribution of measles cases by time of rash onset. A map was drawn to demonstrate the distribution of case-patients by place.
Environmental assessment
During the case-finding activities, we conducted onsite inspections of the health centers, trading centers and households in the outbreak area to observe the crowdedness and exposure risk behaviors among persons.
Laboratory investigations
This was already a confirmed outbreak. By the time of this investigation, blood samples from 16 suspected case-persons had been drawn for laboratory investigations. These investigations were conducted in the Uganda Virus Research Institute (UVRI) EPI laboratory. To declare a measles outbreak in Uganda, at least three out of five samples (60%) collected in an area in the same period must test positive for measles IgM.
Hypothesis generation
For hypothesis generation, we collected data from a random sample of 50 suspected case-patients using a measles case investigation form. We asked case-patients or their caretakers about potential risk factors for measles transmission occurring between 7 and 21 days prior to symptom onset. The risk factors included; not being vaccinated, visiting crowded places with persons with measles in attendance such as places of worship, schools, parties; visiting a health facility and visiting or receiving a visitor from outside the district. We generated hypotheses about potential exposures based on findings from both descriptive epidemiology analysis and hypothesis-generation interviews.
Case control investigation
We conducted an unmatched case-control study to test the hypotheses, in which we interviewed 100 cases and 100 controls. A control-person was a resident of Kakumiro with no history of fever and rash from February 1– May 30, 2024. Controls were selected from the same village and age category which was within +/- 3 years age difference as the case-patients. We used an interviewer administered questionnaire to interview case-patients and control-persons. We asked case-patients and control-persons about their vaccination status (checking the child’s vaccination card or presence of vaccination scars for confirmation), travel history, receipt of visitors, exposure to measles case-patients including going to gatherings such as schools, water collection points, parties, health facilities with case-patients of measles.
We used conditional logistic regression to identify factors associated with measles.
Vaccine effectiveness
We calculated VE as VE= 1−ORadj×100%, where ORadj is the adjusted odds ratio associated with having received ≥1 dose of measles vaccine.
Vaccine coverage
Vaccine coverage was estimated using the percentage of persons vaccinated among eligible controls.
Ethical considerations
This outbreak investigation was in response to a public health emergency and was therefore determined to be non-research. The Ministry of Health (MoH) gave permission to investigate this outbreak. In agreement with the International Guidelines for Ethical Review of Epidemiological Studies by the Council for International Organizations of Medical Sciences (1991) and the Office of the Associate Director for Science, US CDC/Uganda, it was determined that this activity was not human subject research and that its primary intent was public health practice or disease control activity (specifically, epidemic or endemic disease control activity). This activity was reviewed by the US CDC and was conducted consistent with applicable federal law and CDC policy. §§See, e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq. All experimental protocols were approved by the US CDC human subjects review board (The National Institute for Occupational Safety and Health Institutional Review Board) and the Uganda Ministry of Health and were performed in accordance with the Declaration of Helsinki.
Results
Descriptive epidemiology
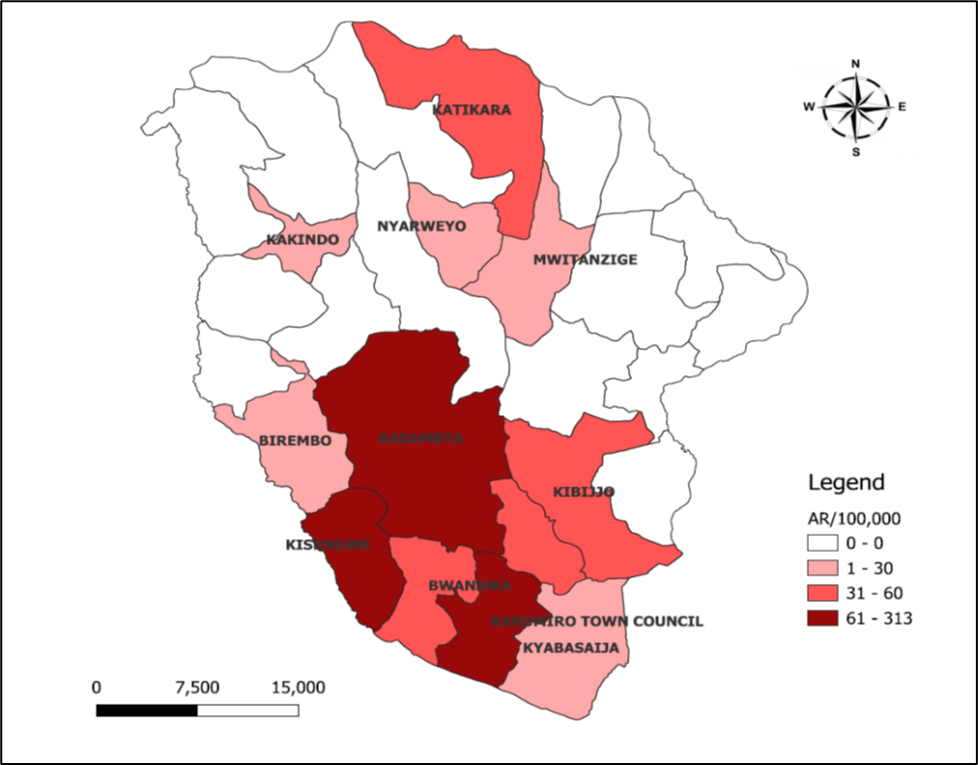
We identified 188 cases of which 6 were confirmed and one died. The overall attack rate was 67/100,000. Medium age was 3 years (IQR: 1.0, 8.5 years). Children <9 months (AR=232/100,000) and those aged 9 months–≤5 years (AR=177/100,000) were the most affected. The majority (75.3%) of case-patients had received at least one dose of MR vaccine. Eleven out of 24 (46%) subcounties were affected. The most affected subcounties were Kisengwe (AR=313/100,000), Kasambya (AR=126/100,000), and Kakumiro town council (AR=110/100,00) (Figure 2).
All the 188 cases (100%) had presented with fever and a rash, 175 (93%) had coryza, 124 (66%) had conjunctivitis, and only 51(27%) had developed cough
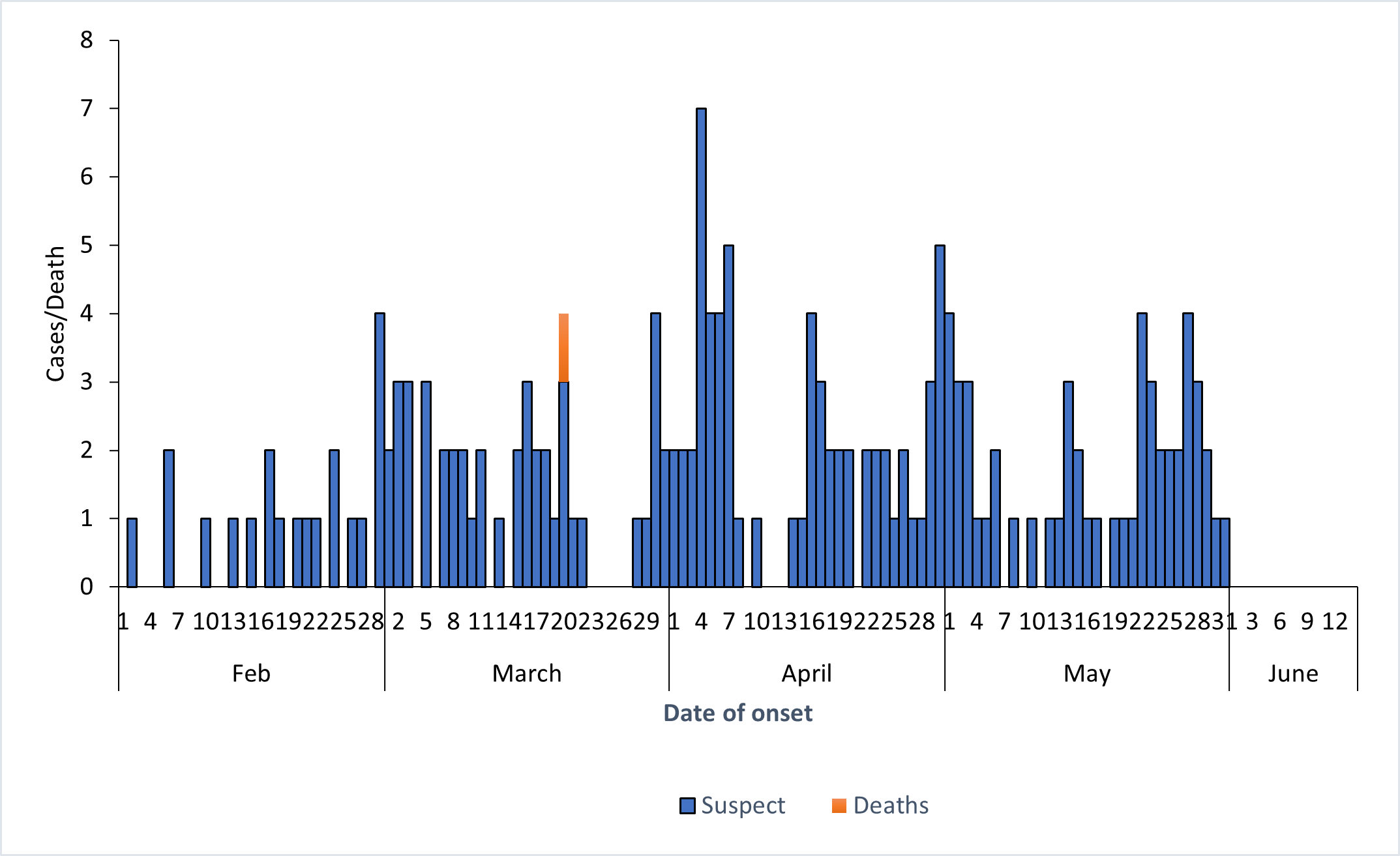
This was a propagated measles outbreak that lasted for about 120 days spanning from February 2 to May 30, 2024. However, no new cases were identified in two incubation periods (Figure 3).
Environmental assessment findings
We observed crowding at health facility OPDs and pediatric IPDs with no form of triage or isolation for measles case-persons in place. At community level, we also observed crowding in gatherings such as churches, markets, and trading centers. Healthy children freely interacted with case-persons having active measles infections.
Hypothesis generation findings
Of the 50 case-patients interviewed for hypothesis generaion,40 (80%) were attending school, 38 (76%) lived in household with > 5 occupants, 29 (58%) reported to always spend >15 minutes at water collection points, 20 (40%) had visited a health facility during exposure period,7 (14%) had not received any measles containing vaccine, 6 (12%) had received visitors in their households during exposure period, and 5 (10%) had travelled outside Kakumiro district.
We hypothesized that the outbreak was propagated by exposure to measles case-patients in any place of congregation such as schools, households, water collection points, and health facilities and lack of vaccination.
Risk factors for infection and transmission among case-patients during an outbreak of measles, Kakumiro District, February–May, 2024
Twenty-one (68%) case patients and 10 (32%) control-persons had never received any MR vaccine (aOR=2.9, 95%CI: 1.1-7.6). Forty-four (93.6%) case-patients and 3 (6.4%) control-persons were exposure to a measles case-patient in a crowded health facility during exposure period (aOR=47, 95%CI: 6.09-369). Forty-two (89.4%%) case-patients and 5 (10.6%) control-persons were exposure to measles case-patient in the same household during exposure period (aOR=9.3, 95%CI: 2.9-30) (Table 1).
Table 1: Risk factors for infection and transmission among case-patients during an outbreak of measles, Kakumiro District, February–May, 2024
| Variable | Cases n (%) |
Controls n (%) |
cOR | (95%CI) | aOR | (95%CI) | |||||||||
| Traveled outside the district during exposure period | |||||||||||||||
| No | 97 | (51) | 94 | (49) | Ref | ||||||||||
| Yes | 1 | (14) | 6 | (87) | 6.2* | (0.7-52.4) |
– |
– |
|||||||
| Exposure to a measles case-patient in a school among school goers | |||||||||||||||
| No | 13 | (65) | 7 | (35) | Ref | ||||||||||
| Yes | 5 | (38) | 6 | (62) | 3* | (0.7-12.6) |
– |
– |
|||||||
| Exposure to a measles case-patient in the neighborhood | |||||||||||||||
| No | 59 | (52) | 55 | (48) | Ref | ||||||||||
| Yes | 41 | (48) | 45 | (52) | 1.2 | (0.7-2.1) |
– |
– |
|||||||
| Never received any MR vaccination | |||||||||||||||
| Yes | 62 | (45) | 76 | (55) | Ref | Ref | |||||||||
| No | 21 | (68) | 10 | (32) | 2.6 | (1.1-5.9) | 2.9 | (1.1-7.6) | |||||||
| Exposure to a measles case-patient in a crowded health facility during exposure period | |||||||||||||||
| No | 56 | (37) | 97 | (63) | Ref | Ref | |||||||||
| Yes | 44 | (94) | 3 | (6) | 25 | (7.5-86) * | 47 | (6.09-369) * | |||||||
| Exposure to a measles case-patient in the same household during exposure period | |||||||||||||||
| No | 58 | (38) | 95 | (62) | Ref | Ref | |||||||||
| Yes | 42 | (89) | 5 | (10) | 14 | (5.1-37) * | 9.3 | (2.9-30) * | |||||||
| Received a visitor in the household during exposure period | |||||||||||||||
| No | 90 | (48) | 99 | (52) | Ref | Ref | |||||||||
| Yes | 10 | (91) | 1 | (9.1) | 11 | (1.4-88) * | 4.2 | (0.3-54) * | |||||||
* Fishers exact test
Vaccine coverage and efficacy
The vaccine coverage which we calculated by formula VE= 1−ORadj×100%, where ORadj is the adjusted odds ratio associated with having received ≥1 dose of measles vaccine was 88% (95%CI: 79%-94%) (Table 2).
Vaccine efficacy estimated as a percentage of persons vaccinated among eligible controls was 65% (95%CI: 13%-91%) (Table 2).
Table 2: Estimation of vaccine coverage and efficacy during measles outbreak in Kakumiro District, February–May, 2024
| Variable | Frequency, n (%) | cOR | (95%CI) | aOR | (95%CI) | |||
| Case | Control | |||||||
| Vaccination status | ||||||||
| No | 21 | (25.3) | 10 | (11.6) | Ref | Ref | ||
| Yes | 62 | (74.7) | 76 | (88.4) | 0.39 | (0.17-0.89) | 0.35 | (0.13-0.91) |
Discussion
Our investigation identified risk factors for transmission of measles as non-vaccination for measles, exposure to a measles case-patient in a crowded health facility during exposure period and exposure to measles case-patient in the same household during exposure period. Children below 9 months and those aged 9m–≤5 years) were the most affected. Vaccine coverage was 88% and vaccine efficacy was 65%.
Non-vaccination for measles was associated with measles infection in this outbreak. Similar findings have been reported in several other outbreak studies in Uganda and other settings (8, 9, 10). Vaccination has been documented to have a protective role in reducing the acquisition, transmission and development of complications from a measles infection (1, 2).
Exposure to or being in close contact with an infected person in crowded health facilities or households propagated this outbreak. These findings are similar to those in a study conducted in Mayuge district in 2016 (11). Measles is one of the world’s most contagious disease, spread by contact with infected nasal or throat secretions (coughing or sneezing) (12). One infected person can transmit the virus to 9 out of 10 unvaccinated persons who get into close contact with his/her infected nasal or throat secretions (12). Measles can be transmitted by an infected person from four days prior to the onset of the rash to four days after the rash erupts (2). The virus can stay active and contagious in the air or on infected surfaces for up to two hours (2, 3).
We reported a case fatality rate much lower (0.5%) than that reported in a study conducted in Kyegegwa that reported a CFR of 16% and one in Lyantonde district which reported a CFR of 4.9% (9, 14). This could be related to the higher MR vaccine coverage including uptake of the recently introduced second MR dose among cases in our study as compared to that in these studies which could have reduced the risk of death among cases. However, globally this CFR is within the documented range of 0.1% to 15% (1, 2).
Children below 5 years of age were more affected compared to those above 5 years with the most affected in this age category being children <9 months. This was comparable to findings from other studies conducted in Uganda in the previous years (6, 11, 12). The findings are also plausible in areas where vaccination coverage is low and under situations of crowding, both which were observed in this investigation (12,16). Higher attack rates among children <9 months of age, an age group below the recommended age for the first dose of a measles containing vaccine demonstrates weaning of the passively transferred maternal antimeasles antibodies from the infants (16). These antibodies wean off between 5 and 11 months of age (16).
The coverage (88%) for a single dose of MR estimated in this study, is lower than the >95% recommendation to achieve herd immunity in a community (1). However, it was comparatively higher than what was reported in studies conducted in Kamwenge district as 75% in 2015, Mayuge district as 68% in 2016 and Lyantonde as 76% in 2017(9, 11, 15). The high coverage could be due to availability of the second dose of MR now as compared to when the other studies were conducted, which has increased the coverage of ≥1 dose (s) of MR among children below 5 years.
Our study estimated vaccine efficacy at 65%, this was lower than what other studies within the country had estimated including in Kamwenge district which had estimated 64% 74% in Mayuge district but in Lyantonde district (9, 11, 15). Several factors influence vaccine efficacy including vaccine quality, number of doses administered, cold-chain failure, and other host factor (16).
Study limitations
There could have been misclassification bias in the selection of controls since none was tested for IgM to rule out incubation of the virus. This could have skewed our findings in the risk factors for transmission of the infection. However, we mitigated this by following a stringent inclusion criterion guided by the case definition. We did not verify every vaccination status reported by checking the immunization cards since others reported having lost or not having them, this could have slightly skewed the estimated vaccination coverage and efficacy. However, we made attempts to verify reported vaccination statuses without cards by checking for a vaccination scar on the left upper arm of the children. We relied on recall of care givers and case-patients to answer most questions and these could have led to some form of recall bias. However, we probed with key events and situations to aid recall.
Conclusion
This measles outbreak was associated with non-vaccination for measles, exposure to a measles case-patient in crowded health facilities and household during exposure period. Children below the recommended age for the first dose of measles containing vaccine were the most affect and the estimated vaccine efficacy in the affected communities was suboptimal.
Recommendations
We recommended to MoH to conduct a supplementary immunization activity targeting children ≥6 months. This was done 2 weeks later. Triaging and isolation of case-persons might help to reduce the spread of measles in future outbreaks. There is also need to develop strategies to improve vaccine efficacy in the district.
Conflict of interest
The authors declare no competing interests
Authors’ Contributions
EOO conceptualized the study idea, collected data, analyzed it, and wrote made the manuscript. PK, JN, PEO conceptualized the study idea, collected data and reviewed the bulletin. IA, RM, BK, DK and ARA supported in editing, and reviewing of the bulletin to ensure scientific integrity. All authors read and approved the final bulletin.
Acknowledgement
We acknowledge the Ministry of Health under Uganda expanded program on immunization (UNEPI), Global Alliance for Vaccine and Immunization (GAVI), and Public Health Fellowship Program (PHFP) for the technical oversight and funds; The District health team of Kakumiro, especially the District surveillance focal person and surveillance team, Immunization and cold chain staff, the village health team structures for the technical support during this investigation; Baylor Uganda for the technical and financial support rendered to the district during this investigation.
Copy right and licensing
All materials in the Uganda Public Health Bulletin are in the public domain and may be used and reprinted without permission; citation as to source; however, is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- Measles vaccines: WHO position paper, April 2017 – Recommendations. Vaccine. 2019 Jan 7;37(2):219–22.
- Wolfson LJ, Grais RF, Luquero FJ, Birmingham ME, Strebel PM. Estimates of measles case fatality ratios: a comprehensive review of community-based studies†. Int J Epidemiol. 2009 Feb 1;38(1):192–205.
- Mahase E. Measles cases rise 300% globally in first few months of 2019. BMJ Br Med J Online. 2019;365:l1810.
- Fournet N, Mollema L, Ruijs WL, Harmsen IA, Keck F, Durand JY, et al. Under-vaccinated groups in Europe and their beliefs, attitudes and reasons for non-vaccination; two systematic reviews. BMC Public Health. 2018 Dec;18(1):196.
- Minta AA. Progress Toward Measles Elimination — Worldwide, 2000–2022. MMWR Morb Mortal Wkly Rep [Internet]. 2023 [cited 2024 Jun 14];72. Available from: https://www.cdc.gov/mmwr/volumes/72/wr/mm7246a3.htm
- Immunization Data [Internet]. [cited 2024 Aug 14]. WHO Immunization Data portal – African Region. Available from: https://immunizationdata.who.int/dashboard/regions/african-region
- Explore Statistics [Internet]. Uganda Bureau of Statistics. [cited 2024 Aug 13]. Available from: https://www.ubos.org/explore-statistics/
- Measles outbreak in Semuto Subcounty, Nakaseke District, Uganda, June–August 2021. IJID Reg. 2022 Dec 1;5:44–50.
- Biribawa C, Atuhairwe JA, Bulage L, Okethwangu DO, Kwesiga B, Ario AR, et al. Measles outbreak amplified in a pediatric ward: Lyantonde District, Uganda, August 2017. BMC Infect Dis. 2020 Jun 5;20(1):398.
- Phadke VK, Bednarczyk RA, Omer SB. Vaccine Refusal and Measles Outbreaks in the US. JAMA. 2020 Oct 6;324(13):1344–5.
- Majwala RK, Nakiire L, Kadobera D, Ario AR, Kusiima J, Atuhairwe JA, et al. Measles outbreak propagated by children congregating at water collection points in Mayuge District, eastern Uganda, July – October, 2016. BMC Infect Dis. 2018 Aug 20;18(1):412.
- Measles [Internet]. [cited 2024 Jun 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/measles
- Thompson AE. Recognizing Measles. JAMA. 2015 Apr 21;313(15):1584.
- Mafigiri R, Nsubuga F, Ario AR. Risk factors for measles death: Kyegegwa District, western Uganda, February–September, 2015. BMC Infect Dis. 2017 Jul 3;17(1):462.
- Nsubuga F, Bulage L, Ampeire I, Matovu JKB, Kasasa S, Tanifum P, et al. Factors contributing to measles transmission during an outbreak in Kamwenge District, Western Uganda, April to August 2015. BMC Infect Dis. 2018 Jan 8;18(1):21.
- Wilmott RW, Bush A, Deterding RR, Ratjen F, Sly P, Zar H, et al. Kendig’s disorders of the respiratory tract in children e-book. Elsevier Health Sciences; 2018.
- Akande TM. A review of measles vaccine failure in developing countries. Niger Med Pr. 2007;52(5–6):112–6.


Comments are closed.