Leptospirosis Outbreak in Kabale District; Uganda, March-July 2017
Authors: Phoebe Alitubeera1, Claire Biribawa1, Benon Kwesiga1; 1Uganda Public Health Fellowship Program
Summary
Between March 2017 and July 2017, 11 cases of leptospirosis were reported among residents of Kabale and Rubanda districts, Uganda. These cases were detected by an acute febrile illness surveillance system which tests all children under five years with fever for other infections including leptospirosis. We conducted an investigation to identify the source and determine scope of the leptospirosis outbreak, its associated risk factors and recommend evidence based control measures. A probable case was any suspected case with Leptospira immunoglobulin M (IgM) confirmed by enzyme-linked immunosorbent assay.
We identified 11 cases and all met the probable definition. Males (attack rate = 5.4/100,000) were more affected than females (attack rate = 5.4/100,000). 64% were under 5 children. The case-control study which involved 11 cases and 44 controls showed that 55% (6/11) of cases compared to 18% (8/44) of controls reported having had skin wounds or small cuts (OR=5.2, 95% C.I: 1.2-23.1). 100% (11/11) of cases and 48% (21/44) of controls had contact with animals (chi-2: 0.001). Environmental assessment revealed evidence of rodents and domestic animals in most homes. This cluster of leptospirosis cases was associated with exposure to domestic livestock among people with wounds or cuts. We recommended a leptospirosis surveillance system among adults with fever that is not due to malaria.
Background
Leptospirosis is a bacterial zoonotic disease caused by genus Leptospira that is often self-limiting [1, 2]. Clinical illness lasts from a few days to 3weeks or more. 2 phases of illness. Acute phase occurs in the first week and is characterized by abrupt high fever, muscle pain, headache (frontal, retro-orbital), nausea, vomiting, abdominal pain, diarrhea, cough and redness of conjunctiva [1]. Second phase is characterized by prolonged fever, jaundice, bleeding, respiratory insufficiency with or without coughing blood, low blood pressure and mental con- fusion [1].
Case fatality for severe illness is between 5-15% [1]. Confirmation is by Leptospira immunoglobulin M (IgM) in enzyme-linked immunosorbent assay (ELISA) detection kits or polymerase chain reaction assays [1]. Leptospirosis is endemic in tropical areas and is a result of interaction between people, animal reservoirs and environment [1]. Transmission is by non-intact skin or mucous membrane contact with contaminated soil or vegetation or water or urine of infected animals [1]. Incubation period ranges from 2-30 days [1]. In Uganda, the prevalence of leptospirosis is approximately 35% [4].
The morbidity caused by leptospirosis can be controlled by health education of at risk populations, access to safe water, improved sanitation and rodent control. Infectious Diseases Research Collaboration (IDRC) and its partners instituted a pediatric surveillance system for acute febrile illnesses among children under 5 in 24 districts in Uganda including Kabale and Rubanda districts
The guidelines for this surveillance system stipulate that blood samples from patients who have had fever for more than five days but are negative for malaria undergo investigation for leptospirosis plus culture and sensitivity tests for other specified bacteria. Leptospirosis is not routinely screened for in the Uganda health system.
On 7/04/2017, the Uganda Ministry of Health and the Public Health Fellowship Program (PHFP) received notification of 4 confirmed cases and 2 suspected cases of leptospirosis in Kabale district. Presenting complaints of the case persons included: fever, cough, difficulty in breathing, loss of appetite, vomiting, diarrhea and muscle spasms. We therefore set out to conduct an investigation to determine the source and scope of the leptospirosis outbreak, its associated risk factors and recommend evidence based control measures.
Methods
We conducted this study in Kabale district which is found in south-western Uganda. We defined a suspected case as having abrupt onset of fever but negative for malaria, with any of muscle pain, headache, cough, flu, swelling of the periorbital region, nausea, abdominal pain, vomiting, diarrhea, respiratory insufficiency or mental confusion from 4/01/2017 in a resident of Kabale or Rubanda districts.
A probable case was any suspected case with Leptospira immunoglobulin M (IgM) confirmed by enzyme-linked immunosorbent assay. A confirmed case was any probable case with positive poly- merase chain reaction (PCR) for Leptospira. We actively sought out and interviewed case-persons using a case investigation form. Furthermore, we interviewed parents, care- takers, siblings of the case-persons who met the suspected case definition and proceeded to have them tested for Leptospira IgM using ELISA test kits with the help of a lab technician.
We got a total of 11 cases from Kabale and Rubanda districts. We then conducted descriptive analysis using time, place and person characteristics and generated hypotheses. We conducted a case-control study selecting neighborhood controls for the cases at a ratio of 1:4 to test the hypotheses. We collected venous blood samples in vacutainers from the suspected case persons. Using enzyme-linked immunosorbent assay for Leptospira immunoglobulin M (IgM), we tested the samples.
Results
Males (attack rate = 5.4/100,000) were more affected than females (attack rate = 5.4/100,000). 64% were under 5 children (age specific attack rates not calculated- no population estimates). The median age was 3 years (IQR: 8) and the average age was 5.9 years (S.D: 7.2).
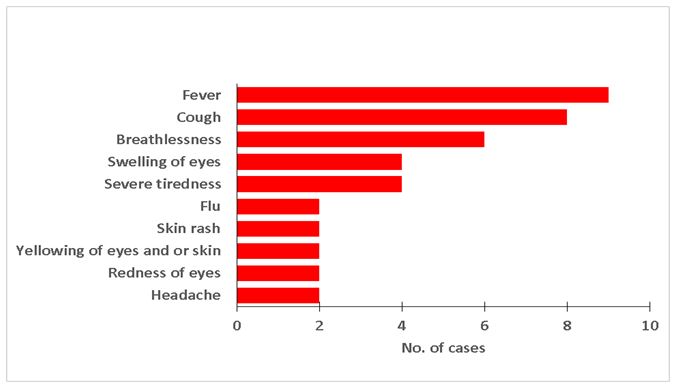
Majority of the case persons reported having had fever, cough and difficulty breathing while slightly less than half reported swelling of eyes and severe tiredness (Figure 1). Case persons were managed on antibiotics: ceftriaxone and gentamicin. 10 (attack rate= 4.3/100,000) were from Kabale district and 1 (attack rate= 0.8/100,000) from Rubanda district. Kabale district had an attack rate 5times that of Rubanda district
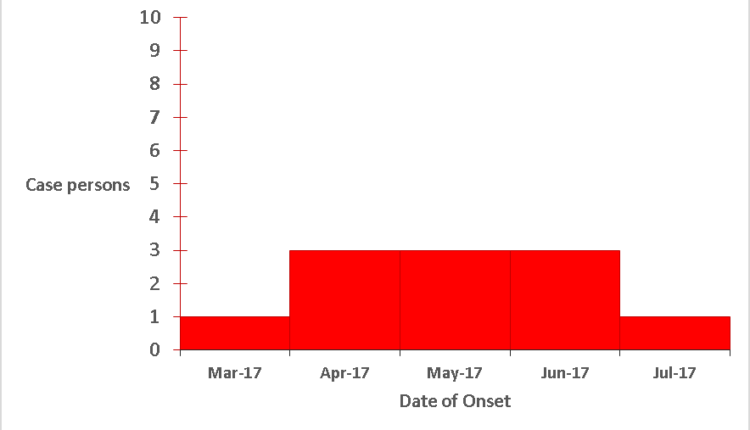
The primary case was identified in March. During April there was an increase in cases which was sustained up to June. In July, there was a drop in cases (Figure 2)
All case-persons had participated in activities that brought them into contact with soil and over half had had wounds in the preceding 3 months (Table 1).
Table 1 Distribution of possible exposure factors among 11 probable leptospirosis case persons in Kabale and Rubanda, Uganda, March-July 2017
| Exposure | Number(n=11) | Percent |
| Rodents in the house | 10 | 91% |
| Contact with animals | 11 | 100% |
| Had cuts or wounds | 6 | 55% |
| Activities that bring them into contact with soil | 9 | 82% |
| Source of water- stream | 10 | 91% |
| -rain | 1 | 9% |
| Boil drinking water | 3 | 27% |
Cases were more likely to have had wounds. Furthermore, cases were just as likely as controls to have rodents in the house (Table 2).
Discussion
Cases had a high likelihood of having had wounds and having contact with animals. This finding is consistent existing knowledge on transmission of Leptospira [1]. Children were the largest contributor to the count of cases because the surveillance system for acute febrile illness is pediatric centered. Empirical evidence shows that prevalence of leptospirosis does not differ by age [4]. Further- more, there is no similar surveillance system among adults and therefore the count of cases among adults may be underestimated.
The complexity of Leptospirosis and its non- specific clinical presentation make diagnosis difficult thus significantly contributing to underestimation of its burden [6]. The months of March to May comprise part of the wet season for Kabale but due to changing climatic patterns the dry season persists up to now. Therefore there were no re- ported episodes of flooding in the recent past. In addition, while majority of cases had contact with stream water, this particular exposure was protective.
This intriguing finding may be because Leptospirosis has a large variety of exposure factors which interact for infection to occur [6]. It is worth noting that both cases and controls were similarly exposed to rodents. Although rodents are notable reservoirs, Leptospirosis is a complex disease with a wide array of hosts [1, 6]. Fever was the commonest symptom among the cases. Leptospirosis is increasingly recognized as an important cause of non-malarial fever [7].
Conclusion
This cluster of leptospirosis cases was associated with expo- sure to domestic livestock among people with wounds or cuts. Leptospirosis is increasingly significant as a cause of non-malarial fever. There is no leptospirosis surveillance system among adults, the existing leptospirosis surveillance system is only among children under five years.
Public health actions and recommendations
We informed the District Health Team of our findings and emphasized avoidance of animal contact especially when one has skin wounds or cuts e.g. by using protective gear while farming. We also encouraged health education about leptospirosis among both health workers and the affected communities. We recommend a leptospirosis surveillance system among adults with fever that is not due to malaria. A community case definition for leptospirosis should be included in the routine Integrated Disease Surveillance Guidelines.
References
- Heymann, D.L., Control of communicable diseases manual. 6448: American Public Health
- Pagès, F., et al., Investigation of a leptospirosis outbreak in triathlon participants, Réunion Island, 2013. Epidemiology & Infection, 144(3): p. 661-669.
- de Vries, S.G., et al., Leptospirosis in Sub-Saharan Africa: a systematic review. International Journal of Infectious Dis- eases, 28: p. 47-64.
- Dreyfus, A., et al., Leptospira Seroprevalence and Risk Factors in Health Centre Patients in Hoima District, Western Uganda. PLoS neglected tropical diseases, 6456. 10(8): p.


Comments are closed.