Improving HIV testing kits inventory management in a high-volume testing laboratory, Uganda, 2023
Authors: Priscilla Atim1,2*, Samuel Gidudu1, Bernard Ssentalo Bagaya2,3, Andrew Kambugu2, Kwabena Sarpong2, Bosco Kafufu2, Benedict Kiwanuka2, Betty Natukunda2, Edith Nekesa2, David Okiror2, Lucy Apeduno2, Audrey Nimwesiga2, Lilian Bulage1, Alex Riolexus Ario1 Institutional affiliations: 1Uganda Public Health Fellowship Program, National institute of Public Health, Kampala Uganda, 2Infectious Diseases Institute, Makerere University, Kampala, Uganda, 3Department of Immunology and Molecular Biology, Makerere University, Kampala, Uganda *Correspondence: Tel: +256774505696 Email: patim@uniph.go.ug
Summary
Background: An evaluation of the HIV-testing kit inventory management in a high-volume testing laboratory showed 22% stock card inaccuracy, 33% stockout of HIV test-kits with a stock out trend of at least one HIV-testing kit every month from July 2022 to March 2023. This consistent stockout of HIV-testing kits resulted in delay in issuing laboratory test results and complaints from clinicians and patients. We conducted a continuous quality improvement (CQI) project to reduce stock out rates of HIV-testing kits from 33% to 0% from March to July 2023 and provided a case study for scaling up the improvement to other test kits inventory in the laboratory.
Methods: We analyzed the laboratory’s HIV-testing kit inventory data to serve as our baseline data. We formed a CQI team in the laboratory to coordinate the implementation of activities. We identified gaps in the inventory system based on analysis of the baseline data. We identified the root cause of the gaps in the system using the “five whys” method. We proposed interventions to identified gaps in the system and ranked the proposed interventions. Based on the proposed interventions, we identified tools to implement the suggested changes. We came up with measurable quality indicators that were used to monitor effectiveness towards improving the HIV test-kits inventory system and evaluated the project at the end of 5 months. We used a Plan Do Check Act (PDCA) cycle to plan and test the implemented interventions in the laboratory.
Results: The root cause for stockout was lack of a standardized reorder system. The implemented changes included: Standardizing the reorder system to use minimum stock levels, creating a consolidated tracking tool, and assigning a focal person. The quality indicator to measure progress towards reducing the stock out rates was the percentage of HIV test-kit stock out in the laboratory. The interventions reduced HIV test-kit stock out from 33% to 0% during May to July 2023.
Conclusion: A quality improvement approach improved inventory management in the short term. The improvement was attributed to standardizing the reordering system, creating a consolidated inventory tracking tool, and assigning a focal person to monitor stock. We recommend continuous monitoring that enables sustainable inventory management.
Introduction
Access to accurate and timely HIV testing is critical for early diagnosis and treatment as the HIV/AIDS epidemic continues to impact populations worldwide (1). Globally, 19% of people living with HIV are undiagnosed and unaware of their status, which is higher than the United Nations Program on HIV/AIDS (UNAIDS) target of 5% (2). Although testing has increased over the years, many people in sub-Saharan Africa are still unaware of their HIV status (3). The effectiveness of HIV testing relies heavily on adequate supply and accurate inventory management of testing kits in health facilities offering this service and adherence to testing algorithms (4)(5).
An effective inventory system is essential to ensure the availability of HIV testing supplies (6). Poor inventory management practices lead to out-of-stock testing kits, which is a common occurrence in healthcare facilities in sub-Saharan Africa (7). In Uganda, from April to September 2023, the average monthly stock-out in government facilities for SD Bioline test kits was 36%, HIV Determine 31% and Stat-pak 24%, with the overall average stockout of 27% above the of 5% target (8). These stock-outs resulted in delayed results release, which was a common complaint among clinicians. The objective of this CQI was to identify the factors leading to laboratory stockouts of HIV test kits from March to July 2023 and to reduce stockout for HIV test kit types to 0%.
Methods
Project implementation setting and design
From March to July 2023, the quality improvement team conducted a project in a high-volume HIV testing laboratory in Kampala. The team chose this laboratory as one of the case study sites because of frequent experienced stockouts of HIV test kits. The goal was to eliminate stockouts for HIV test kit types entirely. To achieve this, the team applied the Plan Do Check Act (PDCA) model, formed a continuous quality improvement team, conducted baseline data collection, identified measures, implemented necessary changes, and monitored the changes using a quality improvement indicator (9,10). The continuous quality improvement model aimed to identify the factors that led to laboratory stockouts of HIV test kit from March to July 2023 and generate interventions for improvement. A final assessment was conducted in July 2023 to assess the improvement.
Ethical approval
We received permission from the laboratory management to carry out this project in the laboratory. Additionally, CDC determined that this investigation was a quality improvement project whose primary intent was to improve HIV testing kit availability and therefore it was classified as non-research.
Problems associated with HIV-testing kit stock out in the laboratory, July 2022-March 2023
The laboratory generally used a manual inventory management system with tools such as stock cards, requisition forms, order forms, and delivery notes.
Ordering system challenges
The common challenge with the inventory ordering system was that there was no defined system for laboratory technologists to send orders for HIV-testing kits to the accounting assistant, which posed a challenge in tracking orders.
Procurement Challenges
While the facility had procurement guidelines for laboratory supplies, including HIV testing kits, the laboratory did not have a procurement plan for them. Additionally, it took an average of 1-2 months for the laboratory to receive supplies of HIV testing kit. As a result, the procurement department had no established procurement cycle and lead time.
Documentation Challenges
The inventory tracking system was largely manual, and, although the average accuracy of the stock cards was 78%, there were problems and errors when filling them. In addition, the available data on order numbers and expiration dates of orders received was limited.
Baseline assessment of inventory management systems for HIV-test supplies
Quantitative Results
Availability status of Rapid HIV-testing kits
At the time of the physical count, 67% of HIV testing kits were available. This was a proportion of available kits at the time of stock assessment N=9.
Stock out of HIV-testing kits
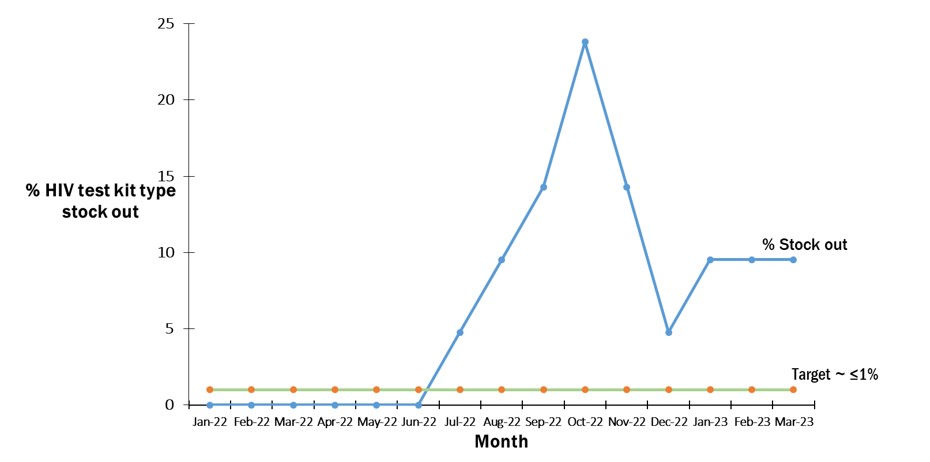
From June 2022 to March 2023, the laboratory experienced stockouts of at least one of the HIV-testing kits in the last six months (Figure 1). The highest peak of stock out (24% stockout) occurred in October 2022, indicating a critical period that required attention.
Data accuracy of stock cards
The stock cards documentation practice was good with an average accuracy of 78% for the HIV-testing kits.
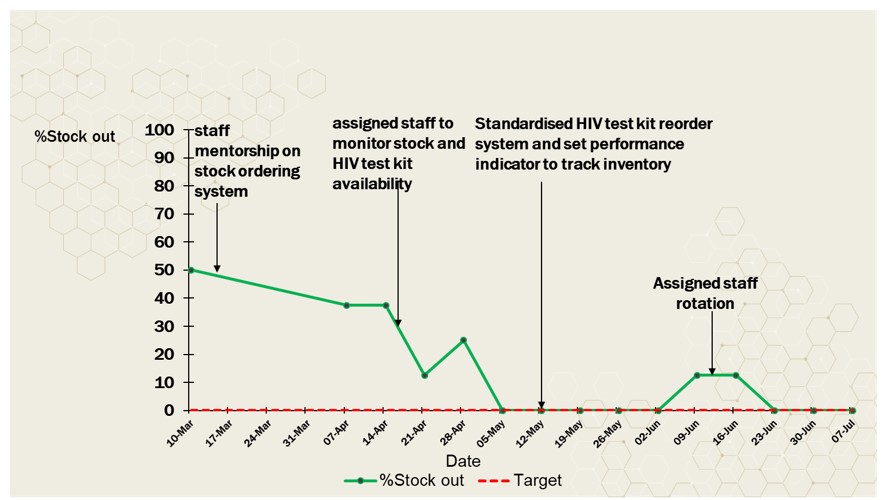
Interventions: We proposed the following changes to address the issues identified. The proposed changes included: standardizing the reordering and restocking system, assigning a focal person to monitor inventory stock levels, and developing a consolidated tool to track the quantity of HIV-testing kits.
Final evaluation of the quality improvement project
After implementing the QI project in the high-volume HIV testing laboratory, the stockout of HIV-testing kits was reduced to 0%.
Discussion
The WHO guidelines emphasize the role of HIV testing and counseling services as an entry point to diagnosis and in facilitating access to appropriate HIV prevention, treatment and care (13). Therefore, scaling up HIV testing plays an important role in HIV control in sub-Saharan Africa (14). Proper inventory management of HIV testing kits is critical to ensure timely availability of testing kits (7). In this regard, we implemented a quality improvement project at a high-volume testing laboratory in Kampala between March and July 2023. Overall, our Quality Improvement Project (QIP) demonstrated an improvement of HIV-testing kit availability at a high-volume testing laboratory in Kampala from 67% to 100%. This was attributed to the implementation of a standardized reordering system with tracking tools and the assignment of a trained focal person to monitor the inventory of HIV testing kits. The formation of a quality improvement team, which included the laboratory’s quality manager, store staff and laboratory technologists who provided bi-weekly reports to management, was also a key component in this project as it maintained the implementation of the QIP and encouraged staff to take a proactive measure like placing timely orders to avoid stock-outs. As trained staff were transferred to other departments in the laboratory, stock-outs increased, demonstrating the importance of trained staff to monitor stock. Teamwork within the QIP team may have resulted in improved reporting of HIV testing kit inventory status. In addition, the use of appropriate data collection tools contributed to improved availability of HIV-testing kit. Training the team on the standardized reordering system also contributed to improved availability of HIV-testing kit.
Study limitations
We did not directly involve all laboratory staff in this quality improvement (QI) project. Consequently, the success of the project may be impacted if key staff members are no longer available, such as when they transfer to another department without adequately training new personnel, or when new staff members join without receiving proper orientation.
Conclusion and recommendations
The CQI project resulted into no stockout of HIV-testing kits in the laboratory during the entire study period. Improving the availability of HIV-testing kits was associated with standardizing of the HIV testing kit reordering system and the assigning trained focal staff to monitor stock. We recommend the laboratory to institutionalize the standardized reordering system to sustain the improvement and expand the strategies to include other test kits used in the laboratory.
Conflict of Interest
The authors declare that they had no conflict of interest.
Authors contribution
AP, designed the study and contributed to data analysis. AP led the writing of the bulletin. SG, BSB, AK, KS, BK, BK, BN, EN, DO, LA, AN, LB, and ARA participated in bulletin writing and review to ensure scientific integrity and intellectual content. All authors contributed to the final draft of the bulletin. All authors read and approved the final bulletin.
Acknowledgements
The authors are indebted to the Infectious Diseases Institute, and Uganda Public Health Fellowship for the technical support during the execution of the project and article writing. We thank Laboratory Leadership Fellowship Program cohort 2023 Fellows for the technical support during the execution of this investigation.
Copyright and licensing
All materials in the Uganda Public Health Bulletin are in the public domain and may be used and reprinted without permission; citation as to source; however, is appreciated. Any article can be reprinted or published. If cited as a reprint, it should be referenced in the original form.
References
- Frescura L, Godfrey-Faussett P, Feizzadeh A A, El-Sadr W, Syarif O, Ghys PD. Achieving the 95 95 95 targets for all: A pathway to ending AIDS. PLoS One. 2022;17(8):e0272405.
- UNAIDS. Global HIV & AIDS statistics — Fact sheet. Fact Sheet 2023 [Internet]. 2023;1–6. Available from: +
http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- Sibanda EL, Taegtmeyer M. Inequalities in uptake of HIV testing despite scale-up. Lancet Glob Heal. 2020 Jun;8(6):e744–5.
- Tania C, Doug T, Greet B, Katrien F, Lut VD. Performance of a Rapid and Simple HIV Testing Algorithm in a Multicenter Phase III Microbicide Clinical Trial. Clin Vaccine Immunol [Internet]. 2011 Sep 1;18(9):1480–5. Available from: https://doi.org/10.1128/CVI.05069-11
- Lule F, Rajab K, Banzimana S, Asingizwe D. Assessing determinants of the availability of HIV tracer commodities in health facilities in Wakiso District, Uganda. J Pharm policy Pract. 2024;17(1):2306846.
- Lugada E, Ochola I, Kirunda A, Sembatya M, Mwebaze S, Olowo M, et al. Health supply chain system in Uganda: assessment of status and of performance of health facilities. J Pharm Policy Pract [Internet]. 2022;15(1):58. Available from: https://doi.org/10.1186/s40545-022-00452-w
- Bekele A, Gemechu F, Ayalew M. Assessment of HIV Rapid Test Kits Inventory Management Practice and Challenges in Public Health Facilities of Addis Ababa, Ethiopia. Integr Pharm Res Pract [Internet]. 2022 Mar 25;11(null):85–94. Available from: https://www.tandfonline.com/doi/abs/10.2147/IPRP.S356134
- MOH. Facility Tracer Medicines Stock Status Summary Report September 2023. 2023;(September):79–81.
- Nguyen V, Nguyen N, Schumacher B, Tran T. applied sciences Practical Application of Plan – Do – Check – Act Cycle for Quality Improvement of Sustainable Packaging : Appl Sci [Internet]. 2020;10(6332):1–15. Available from: doi:10.3390/app10186332
- Taufik DA. PDCA Cycle Method implementation in Industries: A Systematic Literature Review. IJIEM – Indones J Ind Eng Manag. 2020;1(3):157.
- Kits HIVT. Logistics Indicators Assessment Tool ( Liat ) Hiv Test Kits. 2008;(June):1–44. Available from: https://pdf.usaid.gov/pdf_docs/Pnade735.pdf
- Agency U. The Logistics Handbook: A Practical Guide for the Supply Chain Management of Health Commodities. USAID | Deliv Proj Task Order 1 [Internet]. 2011;174. Available from: http://deliver.jsi.com/dlvr_content/resources/allpubs/guidelines/LogiHand.pdf
- WHO. Consolidated guidelines on HIV testing services. N C Med J [Internet]. 2019;53(1):55. Available from: https://www.who.int/publications/i/item/978-92-4-155058-1
- Mannoh I, Amundsen D, Turpin G, Lyons CE, Viswasam N, Hahn E, et al. A Systematic Review of HIV Testing Implementation Strategies in Sub-Saharan African Countries. AIDS Behav. 2022 May;26(5):1660–71.


Comments are closed.