Fatal Pneumonic Plague in Zombo District, West Nile, Uganda, March 2019
Authors: Doreen N. Gonahasa1, Winifred Amia1, Bernadette M. Basuta1, Be-non Kwesiga1, Alex R. Ario1 Affiliations: 1Uganda Public Health Fellowship Program, Ministry of Health, Kampala, Uganda
Summary
On 6 March 2019, the Public Health Emergency Operations Centre (PHEOC) received notification of a female adult patient admitted at Warr Health Center III in Zombo District presenting with symptoms of pneumonic plague. The patient tested positive for plague by Rapid Diagnostic Test (RDT). We investigated to determine the scope of the outbreak, the mode of transmission and recommend control measures. We identified 1 suspected and 1 confirmed case-patient, both females aged 35 years. Both case-patients (100%) had signs and symptoms consistent with pneumonic plague. RDT, culture and serology tests were positive of the one case-person tested. The surviving case-patient had been taking care of a sick relative brought in from Congo with similar symptoms who later died. In total, three of the patient’s relatives are reported to have died, two while in Congo one in Uganda. Results suggested that plague was imported from Congo and delay in seeking healthcare resulted in death of one case-person. Timely response to the outbreak was effective in minimizing its spread. We recommended the following; continuous surveillance especially at border points, and strengthening collaboration during outbreaks between Uganda and the Democratic Republic Congo Ministries of Health.
Background
Plague, which is one of Uganda’s priority zoonotic diseases has three possible presentations; bubonic, septicemic and pneumonic. The incubation period of plague ranges between 1-7 days (1) with variation of 2-8 days for bubonic plague and 1-4 days for pneumonic plague (1). If not quickly and appropriately treated plague is fatal.
Although more recently there are some sporadic cases, improved sanitation has limited the scale of epidemics to focal outbreaks. Advancement in diagnostics and access to appropriate antibiotic therapy have also substantially reduced case-fatality rates. Despite the decrease in human plague cases, plague bacteria continue to circulate in enzootic hosts and their fleas within plague-endemic areas including Democratic Republic of Cong (DRC) and the West Nile region of Uganda, with up to 100% fatality rate of the pathogen for untreated cases (2). West Nile is a densely populated remote area near the borders of DRC and South Sudan.
On 6 March 2019, PHEOC received notification of a female adult patient presenting with fever, cough in blood and difficulty in breathing admitted at Warr Health Center III in Zombo District. The patient tested positive for plague by RDT. We investigated to determine the scope of the outbreak, the mode of transmission, and recommend control measures.
Methods
We defined a suspected bubonic plague case as onset of swollen lymph nodes with fever or chills; and a suspected pneumonic plague case as onset of at least 2 of the following: cough (bloody or wet), chest pain, difficulty in breathing or fever in a resident of Democratic Republic of Congo (DRC) or Zombo District between 1 February and 31 March 2019. A confirmed case was as a suspected case positive for Yersinia pestis by culture or serological tests. We gathered data and information through active case finding and reviewed medical records. We conducted descriptive epidemiology to describe the time, place and person characteristics of the case persons.
Results
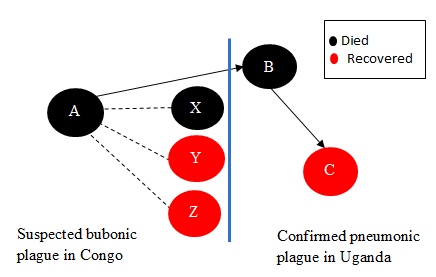
We identified 1 suspected bubonic plague case (Case-patient A; fatal) in DRC and 2 suspected pneumonic plague cases (1 fatal) in Zombo, Uganda. On February 26, Case-patient A (4-year-old boy whose father was Congolese) was buried in DRC near the Uganda border after reportedly succumbing to bubonic plague. Case-patient B (35-year-old mother to Case-patient A) fell ill with suspected pneumonic plague while attending to Case-patient A. It was reported that she did not seek any medical attention while in DRC. Case-patient A was brought by family members to a health facility in Uganda on February 28, but died on arrival probably because the disease had progressed due to delay in receiving treatment.
On March 4, Case-patient C (35-year-old sister to Case-patient B), who attended to Case-patient B while she was ill presented with pneumonic plague symptoms. She tested plague-positive by RDT. Further tests by culture were presumptive positive and serology was positive. She was managed at the health facility on antibiotic treatment until recovery. A total of 114 contacts were traced and given antibiotics as prophylaxis. No additional cases were reported.
Further investigations revealed that in the same family three other people were reported to have suffered from bubonic plague in DRC including one fatality. In total, six family members are recorded to have been affected by the outbreak, four while in Congo (Atungkulei, Mahagi District, Ituri Province) and two in Uganda.
Discussion
This was a mixed bubonic and pneumonic plague outbreak affecting DRC and Warr sub-county, Zombo District. Person, place, and time descriptive evidence showed that the outbreak was associated with travel to the neighbouring DRC where there was already an ongoing outbreak and getting in close contact with an infected case-person. The fatal case was associated with delay in seeking for treatment. Plague has been present for decades in the most southern part of West Nile region in Okoro County, Zombo District and Vurra County, Arua District. Plague hotspots are in the westernmost part of West Nile above the Rift Valley escarpment where elevation is generally >1300m and temperature is relatively low and rainfall high compared to surrounding low lands (2) and this is similar to the Madagascar study where hotspots of pneumonic plague were found in the north-eastern central highlands whose median elevation was within 1200m to 1300m (3).
The case-persons affected were both females of 35 years and this is consistent with the Madagascar reports where pneumonic plague was more frequent in adults of 30 years and above (3). Similar studies in Ugandan however reported a median age of 11 years (range 1-70) years although majority of the case-persons above 15 years were women (2). Also in the Madagascar study, persons >35 years of age were showed to have a moderately higher risk for death than did persons <35 years of age (3). Pneumonic plague has been commonly documented in adults because of participation in funeral ceremonies and attention given to plague patients including taking care of hospitalised relatives (3), this was true for this outbreak.
There was delay in diagnosis due to delay in seeking for treatment for the case-person that died in this outbreak. High frequency of pneumonic plague in adults has been attributed to delayed diagnosis of bubonic plague which then progresses to secondary pneumonic plague. In this outbreak, the delay in seeking care might have been caused by long distance travel from DRC to Uganda. In this outbreak, pneumonic plague case fatality rate (CFR) was 50% and the case per-son had history of travel to the DRC. This is consistent with studies in Madagascar where death was more common for pneumonic case-patients who had recently travelled to a plague-endemic region. Field investigations in DRC and India reported to have found that index case-patients usually travelled long distances (50–200 km) between probable places of infection and their home villages. Recent trips (i.e., trip in a plague-endemic area for 10 days before reporting) has been reported to be strongly (2 fold) associated with pneumonic plague (3). Usually delay in treatment from day 2 up to day 5 has been shown to dramatically increase death rates for both bubonic and pneumonic plague. The average duration from full onset of pneumonic plague to death is 1.9 days (3). In this outbreak however, there was no information on the date of onset of clinical signs of the case-person that died.
The confirmed case was Rapid Diagnostic Test positive, culture presumptive positive, and serology positive. Plague RDT has proven to be highly sensitive and can be used in remote settings and is an important preliminary diagnosis tool that has led to early detection of outbreaks and rapid implementation of control measures in multiple countries in Africa. Tools for early detection and treatment, as well as properly trained health workers, are critical to reducing overall plague deaths and progression of bubonic plague to pneumonic plague (3). Diagnostic capability has been shown to provide considerable savings in terms of treatment, chemoprophylaxis, and pesticide treatment (3). This outbreak was contained because there was early detection of plague by the RDT and response in form of isolation and treatment of the suspected case, contact tracing, and prophylaxis administration to contacts, community sensitization and strengthening of the surveil-lance system in the region. The US Centres for Disease Control and prevention (CDC)/Uganda Virus Research Institute Plague (UVRI) program has enhanced plague surveillance in the West Nile region and this is reflected in their aim; to understand the plague ecology and epidemiology in the West Nile Region and develop appropriate diagnostics, patient treatment and care guidelines and effective public health interventions. The program has strived to sensitize communities about plague and put in place an effective surveillance for plague that has resulted into a decrease of plague cases over the years until this outbreak.
Limitations
Although descriptive data suggested that the outbreak originated from the DRC, we could not further verify that claim. Failure to further verify was majorly because of lack of logistical support and limited cross-border collaborations between the DRC and Uganda.
Conclusion and Recommendations
This was a mixed bubonic and pneumonic Plague outbreak likely imported from the DRC and associated with a fifty percent death rate due to delayed health care seeking. The outbreak affected only one family; implying close contact and the contagious nature of pneumonic plague. All contacts were traced, given antibiotic prophylaxis and followed up for a week. None of the contacts developed plague during or after follow-up.
We recommended strengthening of cross-border collaborations between the Ugandan, Democratic Republic of Congo Ministry of Health bodies, and the World Health Organization (WHO) during response to such outbreaks.
References
1. Control of Communicable Diseases Manual [Internet]. [cited 2019 Mar 18]. Available from: https://www.apha.org/ccdm
2. Al JDF et. Patterns of Human Plague in Uganda, 2008–2016 – Volume 23, Number 9—September 2017 – Emerging Infectious Diseases journal – CDC. [cited 2019 Mar 18]; Available from: https://wwwnc.cdc.gov/eid/article/23/9/17-0789_article
3. Al VA et. Trends of Human Plague, Madagascar, 1998–2016 – Volume 25, Number 2—February 2019 – Emerging Infectious Diseases journal – CDC. [cited 2019 Mar 18]; Available from: https://wwwnc.cdc.gov/eid/article/25/2/17-1974_article


Comments are closed.