
Animal Anthrax outbreak triggered by butchering infected carcasses on and or near the pastureland, Kween District, Uganda: January – December 2018
Authors: Fred Monje1, Daniel Eurien1, Esther Kisaakye1, Benon Kwesiga1, Daniel Kadobera1, Alex Riolexus Ario1
Affiliation: 1Uganda Public Health Fellowship Program, Kampala, Uganda
Summary
On 20th April 2018, 7 people were admitted to Health Centre Y, after skinning, carrying and eating a dead cow at Kaplobotwo village, Kween Di-trict. Subsequent epidemiological investigations confirmed human anthrax outbreak and established link to animals. We set out to determine the magnitude of anthrax infection in domestic ruminants; identify possible exposures, and recommend evidence-based control measures. We identified 67 case-herds and line listed 316 animal cases by December 2018. Only Ngenge subcounty (AR/1000=16) in Kween District was affect-ed. Sixty-seven percent (2/3) animal carcasses tested positive by RDT and Gram stain, consistent with B. anthracis. In the case-control study, migration route near or on the pastureland (AOR=5.6, CI=1.9-16); improper carcass disposal sites near or on the pastureland where animals grazed (AOR=2.0, CI=1.1-3.7), and butchering infected animal carcasses on or near the pastureland (AOR=3.8, CI=1.9-7.5) were significantly associated with animal anthrax infection. We recommended immediate vaccination of domestic ruminants at risk against Anthrax followed by annual anthrax vaccinations.
Introduction
On 20th April 2018, 7 people were admitted to Ngenge HCIII, after skinning, carrying and eating a dead cow at Kaplobwotwo village, Kween District. They all presented with blisters, oedema and gram spots that are typical of an anthrax infection. Subsequent epidemiological investigations confirmed human anthrax outbreak and established link to animals. However, the true burden of anthrax in animals and associated factors remained unknown in the district despite the control measures initiated. We conducted an investigation to assess the magnitude of anthrax infection in domestic ruminants; identify possible exposures, and recommend evidence-based control measures.
Methods
We defined a suspected animal case as sudden death with unclotted blood from body orifices in a domestic ruminant from January 2018 to December 2018 in Kween District. A probable case was a suspected case that tested positive for anthrax by the Active Anthrax Detect Rapid Diagnostic Test (AAD-RDT) or microscopy of Gram-stained animal tissue samples.
To identify cases, we reviewed district veterinary anthrax records and conducted active community case finding in the affected sub-county to generate and updated the line list. The variables captured in the line list included the herd number, animal ID, species, breed, date of deaths, method of carcass disposal, vaccination status, signs and symptoms among others.
We described animal cases by animal characteristics, place, and time. We conducted environmental assessment of the anthrax affected areas, including laboratory testing of specimens from dead animals). We conducted a case-control study to compare exposures among case-herds and control-herds, frequency matched by village with a ratio of 1:2.
Results
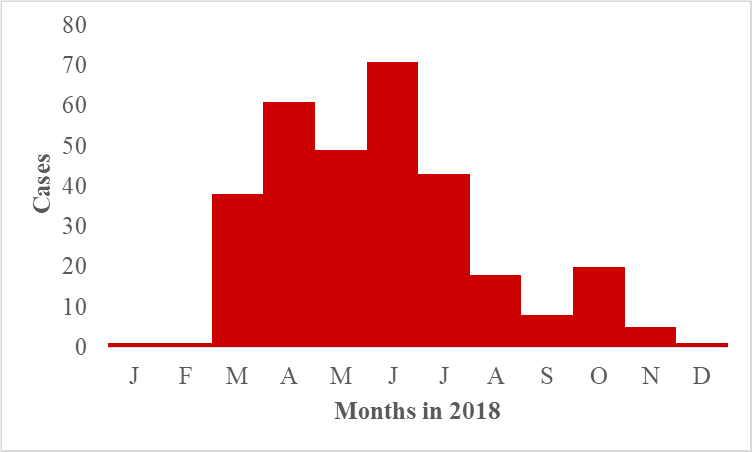
We line listed 316 animal deaths due to suspected anthrax among 67 kraals in Ngenge Subcounty, Kween District. Symptoms of the animals before death included unclotted blood oozing from body orifices (100%), distended abdomen (91%), and sudden deaths (100%). Cattle were the most affected species in Kween District (Attack rate [AR]/1,000 = 2.9, followed by goats (AR/1000= 0.39 and sheep (AR/1,000= 0.10.
The epidemic curve indicated a continuous sustained transmission among the animal population (Figure 1). The animal deaths due to suspected anthrax increased rapidly and peaked in the month of April before declining in May, 2018. It again increased rapidly to another peak in June, 2018 before declining till September 2018. From September 2018, the animal deaths increased to a smaller peak in October 2018. Thereafter, the animal deaths de-creased till December 2018.
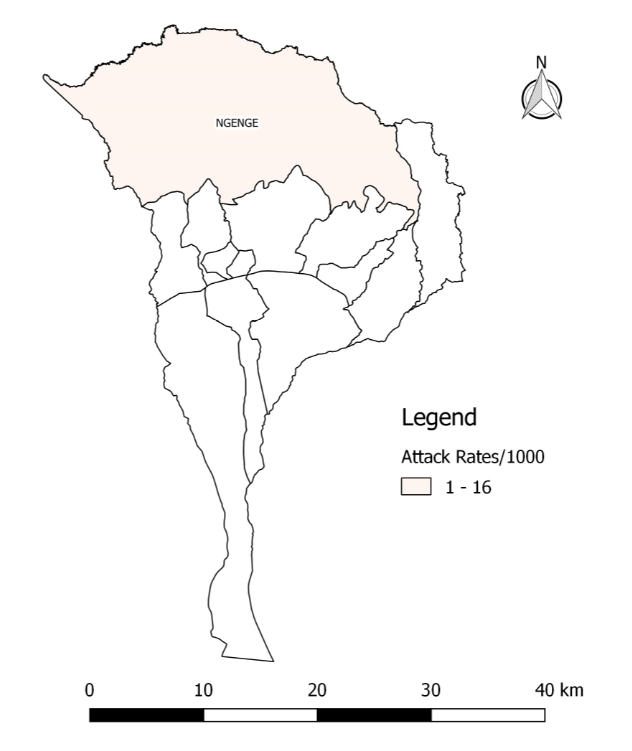
Only Ngenge sub-county was affected (AR/1000=16) (Figure 2).
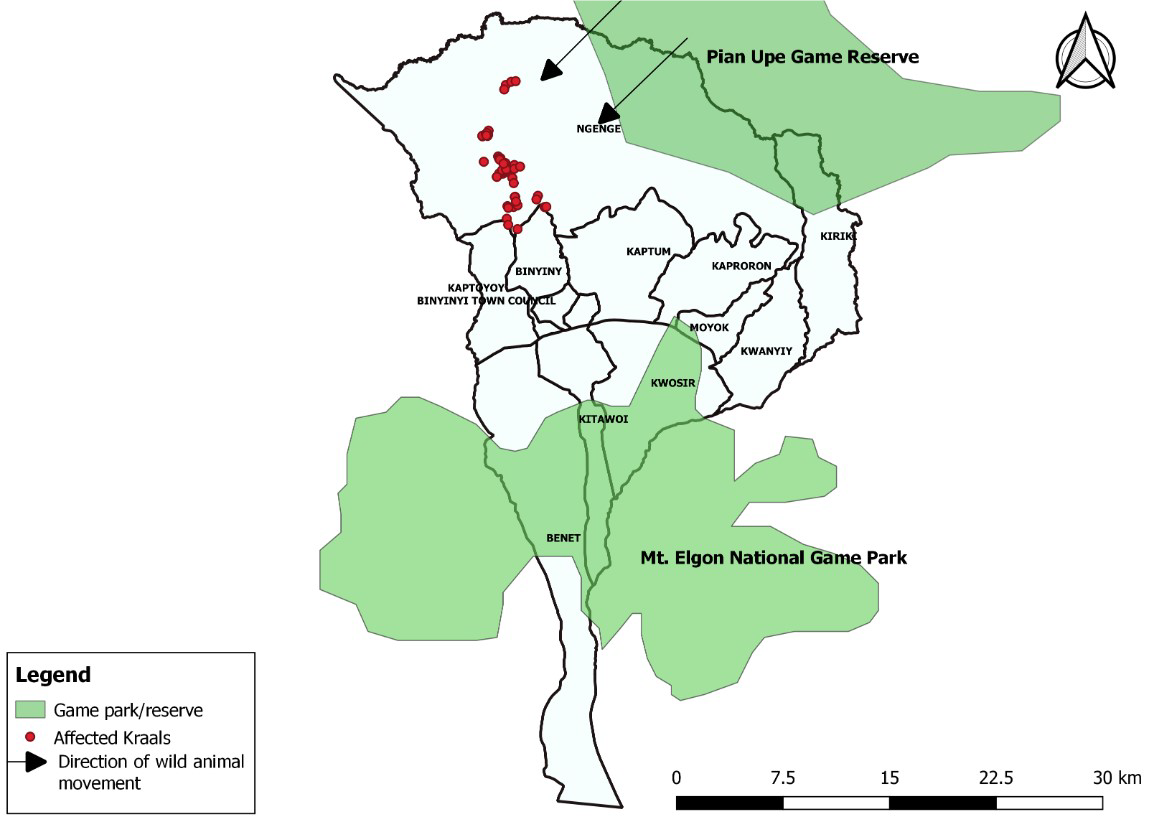
Hypothesis generation interviews
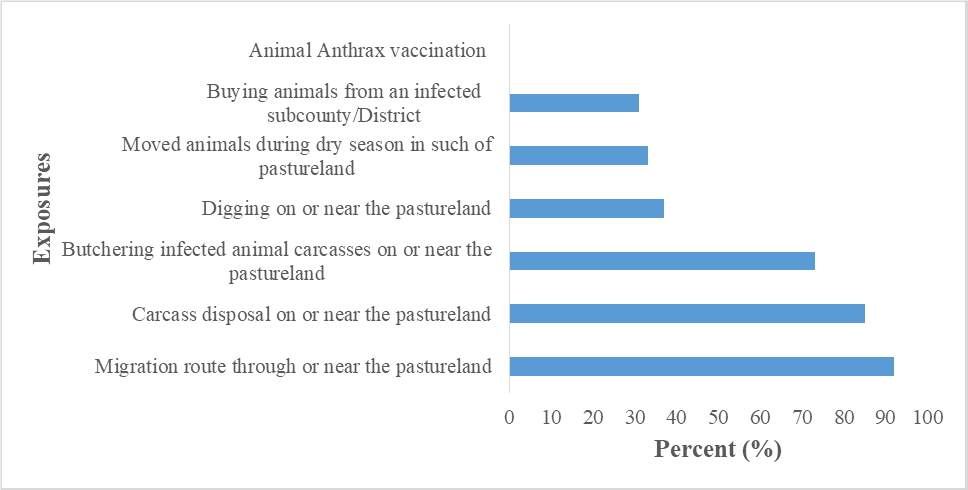
We hypothesized that the outbreak could have been associated with having migration route through or near pasture land where animals grazed, butchering infected animal carcasses on and or near pasture land where animals grazed, and improper disposal of carcasses on and or near pasture land (Figure 4).
Environmental assessment and laboratory findings
We observed communal grazing and remains of the animal carcasses in the grazing field. Migration routes in the pasture land were evident. During the study period, sixty-seven percent (2/3) livestock carcasses tested positive by RDT and Gram stain, consistent with B. anthracis.
Case-control findings
Migration route near or on the pastureland (AOR=5.6, CI=1.9-16); carcass disposal sites near or on the pastureland (AOR=2.0, CI=1.1-3.7), and dead animals slaughtered near or on the pastureland (AOR=3.8, CI=1.9-7.5) were significantly associated with animal anthrax infection.
Discussion
Our epidemiologic, laboratory and environmental findings demonstrated that this was a continuous sustained transmission among animal population associated with infected carcass disposal sites on or near the pastureland; slaughtering anthrax infected dead animals on the pastureland, and migration routes near or on the pastureland. Improper disposal sites of infected carcasses on the pastureland and slaughtering anthrax infected dead animals on the pastureland exposes anthrax spores that contaminate the pastureland posing a risk to cattle, goats, and sheep. The anthrax spores from the pastureland remain viable for decades (1). The spores shed by an animal dying or dead from anthrax usually provide the source of infection of other animals (2). Our findings are consistent with other studies in Bhutan, Asia that pointed out that anthrax infected carcasses led to spread of anthrax and death among animal herds (3). Similarly, findings from Bangladesh indicated that improper disposal of carcass (dead animals) was associated with repeated anthrax infections among animals (4). Further-more, findings in North Dokota, USA also reported that death of suspected anthrax infected animals on the pasture land was associated with anthrax outbreak in animals (5).
Besides, migration routes on the pastureland are paths were people pass along with their animals whether dead or alive usually without veterinary inspection. If the meat from dead animal is infected with anthrax, then it may infect the pastureland along the migration route during transportation due to dripping infected blood of the meat. Similarly, if the live animal dies suddenly due to anthrax during transit, it is likely to be slaughtered or abandoned near the migration route in the pastureland thereby posing a further risk to live animals on that pastureland.
Our findings also revealed that cattle were more affected than goats and sheep. This is possibly due to the fact that cattle are grazers while the goats and sheep are browsers. This is consistent with findings from China that found out that cattle were more affected by anthrax compared to goats because cattle ingest lots of soil from ground when grazing, while goats typically browse on grass only, which makes them less exposed to anthrax spores in soil (6).
Our study had the following limitations; given that an animal dies suddenly, it is possible that some animals dropped dead in the field and nobody noticed them. Additionally, some farmers have many animals hence the possibility of failure to notice absence of some. Failure to account for all anthrax related animal deaths in the area may have resulted into an underestimation of the scope of the out-break.
Conclusions and recommendations
In conclusion, there was evidence of animal anthrax infection in Kween District. Improper disposal of infected carcasses and slaughtering anthrax infected carcasses on or near the pastureland were the main exposures.
We recommended to the Ministry of Agriculture, Animal Industry and Fisheries (MAAIF) and Kween District Local Government to conduct immediate vaccination of domestic ruminants at risk against anthrax followed by annual anthrax vaccinations. Enhanced sensitization of the communities; animal health workers and medical health workers about anthrax exposure was also emphasized.
References
1. Carlson CJ, Getz WM, Kausrud KL, Cizauskas CA, Blackburn JK, Carrillo FAB, et al. Spores and soil from six sides: interdisciplinarity and the environmental biology of anthrax (Bacillus anthracis). Biol Rev [Internet]. [cited 2018 Sep 19];0(0). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/brv.12420
2. World Health Organization, International Office of Epizootics, Food and Agriculture Organization of the United Nations, editors. Anthrax in humans and animals. 4th ed. Geneva, Switzerland: World Health Organization; 2008. 208 p.
3. Thapa NK, Wangdi K, Dorji T, Dorjee J, Marston CK, Hoffmaster AR. Investigation and Control of Anthrax Outbreak at the Human–Animal Interface, Bhutan, 2010. Emerg Infect Dis. 2014 Sep;20(9):1524–6.
4. Hassan J, Ahsan M, Rahman M, Chowdhury S, Parvej M, Nazir K. Factors associated with repeated outbreak of anthrax in Bangladesh: qualitative and quantitative study. J Adv Vet Anim Res. 2015;2(2):158.
5. Mongoh MN, Dyer NW, Stoltenow CL, Khaitsa ML. Risk Factors Associated with Anthrax Outbreak in Animals in North Dakota, 2005: A Retrospective Case-Control Study. Public Health Rep. 2008 May 1;123(3):352–9.
6. Chen W-J, Lai S-J, Yang Y, Liu K, Li X-L, Yao H-W, et al. Mapping the Distribution of Anthrax in Mainland China, 2005–2013. PLoS Negl Trop Dis. 2016 Apr 20;10(4):e0004637.

Comments are closed.