Crimean-Congo Hemorrhagic Fever Outbreak among Livestock Workers, Central Uganda – Aug-Sep 2017
Author: Susan Kizito 1, Paul Okello1, Benon Kwesiga 1, Luke Nyakarahuka 2, Alex Riolexus Ario 1; Affiliations: 1Uganda Public Health Fellowship Program; 2Uganda Virus Research Institute
Summary
On 20 th August , 20 17 Uganda Ministry of Health was notified of two RT- PCR-confirmed Crimean-Congo Hemorrhagic Fever (CCHF) cases from two district hospitals in Central Uganda. Following the alert, a multi-disciplinary National Rapid Response Team (NRRT) responded with the objective of determining the scope, risk factors for the outbreak and recommending evidence based control measures. The case fatality rate was 76.4% and Nakaseke District was the most affect- ed. We also conducted a frequency-matched case-control study which showed that cases had bites/contact with ticks.
Introduction
CCHF is a hazardous viral hemorrhagic disease of epidemic potential (1,2). Today, it is the most widely distributed tick- borne viral disease mainly affecting humans in Africa, Asia and Eu- rope (3,4). CCHF is enzooticaly and epizootically maintained in the environment and transmitted through tick bites or contact with infected animal tissues (1,5). Sick persons present with sudden onset of high grade fever, body weakness, yellowing of eyes. Later on cases present with bleeding symptoms although most go undiagnosed. Up to 40% of infected people die from the disease (6). The spread of dis- ease is believed to be favored by recent global climatic changes like warmer temperatures, grassland vegetation, livestock trade, and movement of migratory birds during summer periods (5,7).
On August 20, 2017 the Uganda Virus Research Institute (UVRI) notiied the Ministry of Health (MoH) of two confirmed Viral Hemorhagic Fever (VHF) cases from two district hospitals in Central Uganda. Both cases presented with high grade fevers and uncontrollable bleeding. Blood samples from the suspected cases were confirmed positive for Crimean – Congo Hemorrhagic Fever by real-time reverse transcriptase Polymerase Chain Reaction (RT- PCR) after excluding Ebola, Marburg, RVF and Sosuga hemorrhagic fevers. The two confirmed case-persons were working in two of central Uganda’s cattle- producing districts – Nakaseke and Kyankwanzi. Following the alert, a multi-disciplinary National Rapid Response Team (NRRT) immediately responded with the objective of determining the scope, risk factors for the outbreak and recommending evidence based control measures.
Methods
We defined a probable case-person as sudden onset of fever for ≥3 days, with spontaneous bleeding or bruising or clinical laboratory evidence of pancytopenia (all unexplained by other causes) in a resident of districts affected by the outbreak from July to August 2017. A confirmed case was a probable case with confir- mation of CCHF by RT-PCR. We identified cases mainly through the review of medical records at district hospitals and active case finding with assistance from local leaders and village health team members. We visited the farms on which the confirmed cases worked and collected ticks and blood samples from some of the animals. We conducted an environmental assessment and carried out a matched-case-control study among asymptomatic male case- person neighbors of similar age-group.
Results
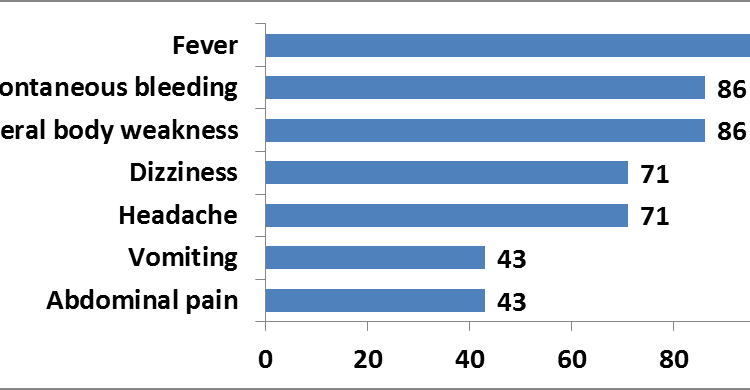
Person distribution: We line-listed 7 adult case-persons, five of which met the probable case definition while two were confirmed. All case-persons (100%) were male. The median age was 26 years, and age range 15 to 87 years. Two probable case-persons died, (Case Fatality Rate = 29%). Clinical presentation of symptoms were: fever (100%), general body weakness (86%), spontaneous bleeding (86%) among case-persons. The high body temperatures were persistent despite use of anti-malarial treatment, antibiotics or anti-pyretic medication. These key symptoms were consistent with Crimean-Congo Hemorrhagic Fever.
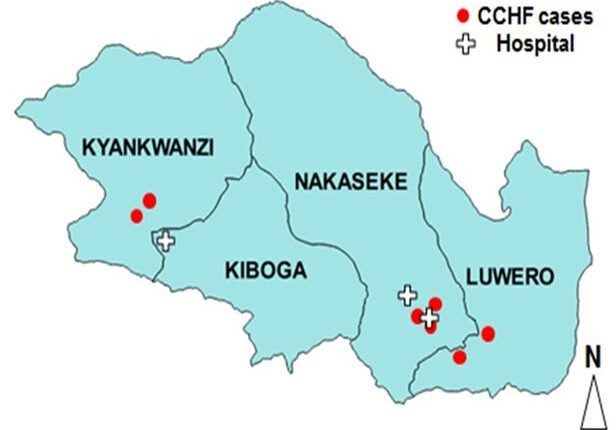
Place distribution: Case-persons were from three districts: two from Kyankwanzi, two from Luwero and three from Nakaseke districts. The spot map shows the place distribution of cases in relation to the location of hospitals were treatment was sought
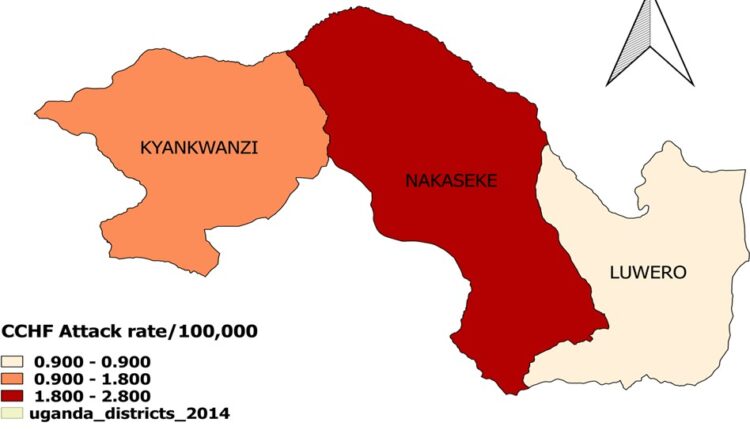
The attack rate (AR) per district shows that Nakaseke had the highest attack rate, (AR=2.8/100,000), followed by Kyankwanzi (AR=1.8/100,000), and Luwero (AR= 0.9/100,000) (Figure 3).
Laboratory analysis of animal specimens: An analysis of blood samples collected from livestock animals on farms were confirmed case-persons worked showed 9 (60%) of blood samples from cattle and 2 (29%) of goat blood samples were CCHF seropositive.
Case-Control Study: We conducted a case-control study in which case-persons were frequency-matched to controls by gender, age-group and neighborhood. Having experienced a tick bite or having squashed ticks with bare hands was trongly associated with acquiring CCHF (ORM-H=11, 95% CI=1.1-112). The other exposure considered were contact with livestock in the last two weeks (OR = 2, 95%CI=0.18-105), having milked livestock with bare hands (OR =0.62, 95%CI=0.10-3.8), contact with raw wild animals meat (OR = 1.39, 95%CI=0.02-15). However, these exposures were not statistically significant.
Discussion
The CCHF outbreak identified in three cattle-corridor districts of Kyankwanzi, Luwero and Nakaseke was most likely due to tick bites. The magnitude of the outbreak was most felt in Nakaseke district although the extent of the outbreak could have been under estimated. Sporadic cases of CCHF will continue to arise be- cause of the favorable multiplication of the virus transovarially and transtadially (8). The virus multiplies in livestock and livestock workers continue to get exposed although not many are seen to be reported. This could be due to limited review of medical records, insufficient clinical history data or inability to carry out diagnostic tests.
There were no reported secondary cases among hospital staff or care takers despite the nature of disease having a high transmission rate. This could be due to Uganda’s growing capacity to man- age and control viral haemorrhagic fevers like CCHF, Ebola and Mar- burg through surveillance, work-force and laboratory capacities as well as the existence of the Emergency Operations Centre to rapidly prevent, detect, respond and control outbreaks t the source.
Conclusion
The CCHF outbreak was a fatal zoonotic viral haemorrhagic disease transmitted by ticks in the 3 districts of central Uganda. Spraying of livestock with pesticides to kill ticks will help to control transmission of disease.
References
- Mourya DT, Yadav PD, Patil DY. Highly infectious tick-borne viral diseases: Kyasanur forest disease and Crimean–Congo haemorrhagic fever in 2014
- Abdelhakam HA, Taha MA. Crimean-Congo hemorrhagic fever (CCHF) in Southern Sudan J Paediatr [Internet]. 2014 [cited 2017 Oct 23];14(1):81–4. Available from: http:// europepmc.org/abstract/med/27493394
- Leblebicioglu H, Ozaras R, Erciyas-Yavuz Emergence of Crimean-Congo hemorrhagic fever. Trans R Soc Trop Med Hyg. 2015;109 (11):676–67
- Dreshaj S, Ahmeti S, Ramadani N, Dreshaj G, Humolli I, Dedushaj Current situation of Crimean-Congo hemorrhagic fever in South- eastern Europe and neighboring countries: a public health risk for the European Union? Travel Med Infect Dis. 2016;14(2):81–91.
- Sutherland LJ, Anyamba A, LaBeaud Emerging and Reemerging Human Bunya virus Infections and Climate Change. 2013
- Fajs L, Humolli I, Saksida A, Knap N, Jelovšek M, Korva M, et al. Prevalence of Crimean-Congo hemorrhagic fever virus in healthy population, livestock and ticks in Kosovo. PloS One. 2014;9(11):e110982.
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic Antiviral Res. 2013;100(1):159–189.


Comments are closed.